Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

15.11 Additional Problems 759
3
4
1
09876543210
(ppm)
Chemical shift (δ)
1
09 8 7 6 5 4 3 2 1 0
(ppm)Chemical shift (δ)
1
09 8 7 6 5 4 3 2 1 0
(ppm)
Chemical shift (δ)
2
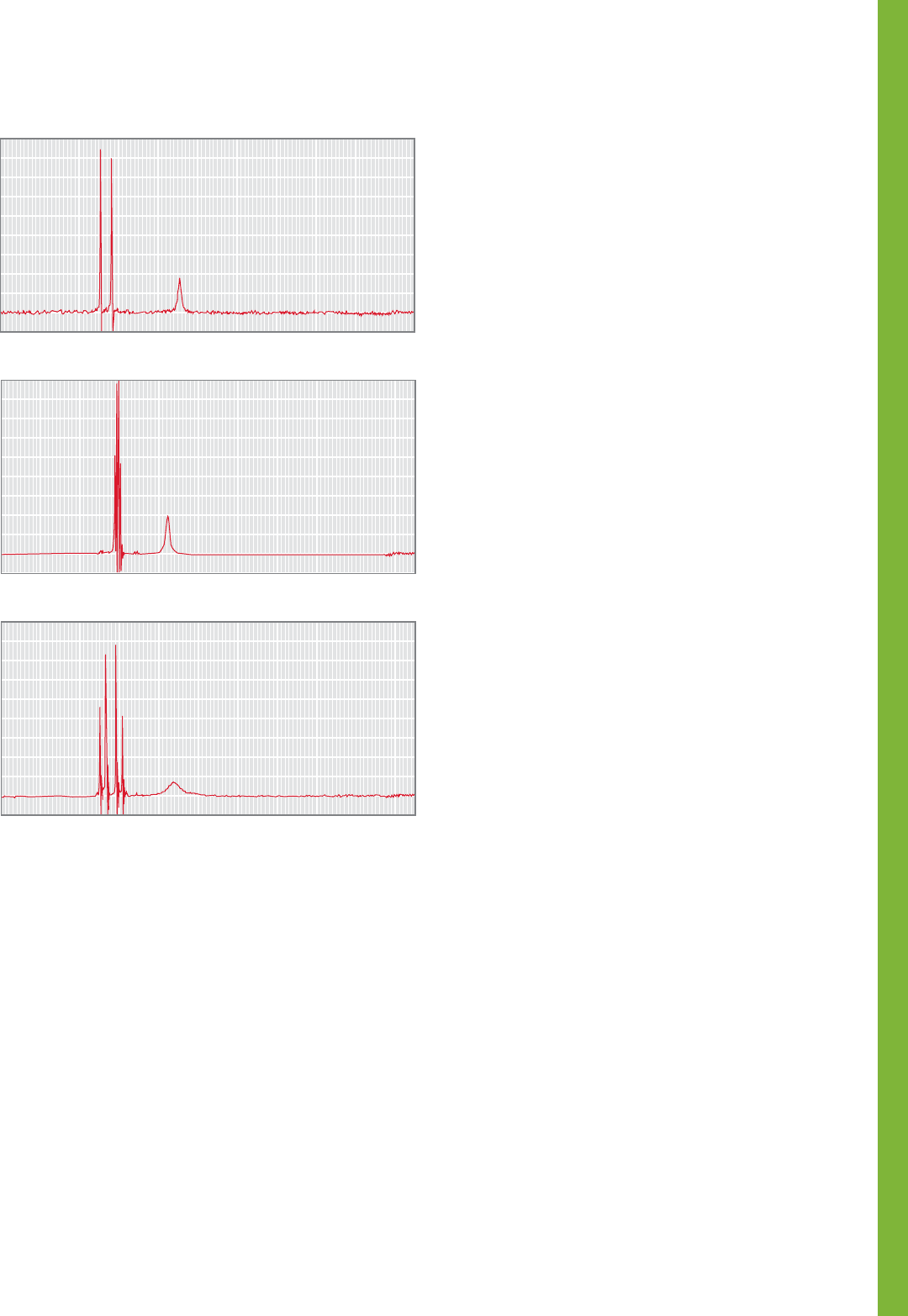
PROBLEM 15.82 Compounds A and B, containing only C,
H, and O, gave 63.15% C and 5.30% H upon elemental
analysis. Their mass spectra do not show a molecular ion, but
freezing point depression data indicate a molecular weight of
150 g/mol. Catalytic hydrogenation of both A and B leads to
C. Spectral data for compounds A, B, and C are summarized
below. Assign structures for compounds A, B, and C and
explain your reasoning.
Compound A
IR (Nujol®): 1845 (m), 1770 (s) cm
1
1
H NMR (CDCl
3
): δ 2.03–2.90 (m, 2H)
3.15–3.70 (m, 1H)
5.65–6.25 (m, 1H)
Compound B
IR (Nujol®): 1825 (m), 1755 (s) cm
1
1
H NMR (CDCl
3
/DMSO-d
6
): δ 1.50–2.10 (m, 1H)
2.15–2.70 (m, 1H)
Compound C
IR (neat): 1852 (m), 1786 (s) cm
1
1
H NMR (CDCl
3
/DMSO-d
6
): δ 1.10–2.30 (m, 4H)
3.05–3.55 (m, 1H)
PROBLEM 15.83 Spectral data for isomeric compounds A and
B are summarized below. Assign structures for compounds A
and B, and explain your reasoning.
Compound A
Mass spectrum: m/z 148 (M, 7%), 106 (8%), 105 (100%),
77 (29%), 51 (8%)
IR (neat): 1675 (s), 1220 (s), 980 (s), and 702 (s) cm
1
1
H NMR (CDCl
3
): δ 1.20 (d, J 7 Hz, 6H)
3.53 (septet, J 7 Hz, 1H)
7.20–7.60 (m, 3H)
7.80–8.08 (m, 2H)
Compound B
IR (neat): 1705 (s), 738 (m), and 698 (s) cm
1
1
H NMR (CDCl
3
): δ 0.95 (t, J 7 Hz, 3H)
2.35 (q, J 7 Hz, 2H)
3.60 (s, 2H)
7.20 (s, 5H)
PROBLEM 15.84 Upon direct photolysis or heating at 220 °C,
the dimer of 2,5-dimethyl-3,4-diphenyl-2,4-cyclopentadien-
1-one (A) yields compound B, mp 166–168 °C. Spectral data for
compound B are summarized on the next page. Deduce the struc-
ture of compound B and explain your reasoning. (You may have
to make an educated guess about the actual stereochemistry of B.)
PROBLEM 15.81 Isomeric compounds A and B have the com-
position C
11
H
12
O
4
. Spectral data are summarized below.
Deduce structures for A and B and explain your reasoning.
Compound A
IR (KBr): 1720 (s), 1240 (s) cm
1
1
H NMR (CDCl
3
): δ 2.46 (s, 3H)
3.94 (s, 6H)
8.05 (d, J 2 Hz, 2H)
8.49 (t, J 2 Hz, 1H)
Compound B
IR (Nujol®): 1720 (s), 1245 (s) cm
1
1
H NMR (CDCl
3
): δ 2.63 (s, 3H)
3.91 (s, 6H)
7.28 (d, J 8 Hz, 1H)
8.00 (dd, J 8 Hz, 2 Hz; 1H)
dd doublet of doublets
8.52 (d, J 2 Hz, 1H)
Compound B
Mass spectrum: m/z 492 (M)
IR (CCl
4
): 1704 cm
1
1
H NMR (CDCl
3
): δ 0.73 (s, 3H)
0.92 (s, 3H)
1.51 (s, 3H)
1.88 (s, 3H)
6.6–7.5 (m, 20H)
760 CHAPTER 15 Analytical Chemistry: Spectroscopy
CD E
NH
2
Cl
CH
3
C
O
116.1
128.8
119.0
126.5
129.5
128.7
134.9
147.7
PROBLEM 15.85 An appreciation of the concepts of elec-
tronegativity (inductive effects) and of electron delocalization
(resonance effects), combined with an understanding of ring
current effects, permits both rationalization and prediction of
many chemical shifts.
(a) The carbons of ethylene have a
13
C NMR chemical shift of
δ 123.3 ppm. Rationalize the
13
C NMR chemical shifts of
the β carbons of 2-cyclopenten-1-one (A) and methyl vinyl
ether (B). (Hint: Think resonance!)
B C
O
OO
MgCl
2
H
2
O
autoclave
230 ⬚C, 2 h
A
OH
(b) The
1
H NMR chemical shifts of hydrogens ortho, meta, and
para to a substituent on a benzene ring can also be correlated
in this way. For reference sake, note that the hydrogens in
benzene appear at about δ 7.3 ppm. Aniline (C) exhibits
three shielded aromatic hydrogens (i.e., δ 7.0), whereas the
other two aromatic hydrogens appear in the normal range.
On the other hand, two of the aromatic hydrogens in ace-
tophenone (D) are strongly deshielded (δ 7.9 ppm), one is
somewhat deshielded (δ 7.5 ppm), and the other two aro-
matic hydrogens appear at δ 7.4. Finally, all five aromatic
hydrogens in chlorobenzene (E) appear at δ 7.3 ppm. Assign
the
1
H NMR chemical shifts of the aromatic hydrogens for
C, D, and E by considering inductive, resonance, and ring
current effects. Additionally, the
13
C NMR chemical shifts
for the aromatic carbons of C and E are shown in color in
the next column. (The
13
C NMR chemical shift for benzene
is δ 128.5 ppm.) Are these
13
C NMR chemical shifts consis-
tent with the
1
H NMR chemical shifts for C and E?
PROBLEM 15.86 Compound A was isolated from the bark of
the sweet birch (Betula lenta). Compound A is soluble in 5%
aqueous NaOH solution but not in 5% aqueous NaHCO
3
solu-
tion. The spectral data for compound A are summarized below.
Deduce the structure of compound A. (Be sure to assign the
aromatic resonances in the
1
H NMR spectrum. Note that J
meta
and J
para
were not observed.) Hint: The pK
a
of phenol is about
10, and that of benzoic acid is about 4.2.
Compound A
Mass spectrum: m/z 152 (M, 49%), 121 (29%), 120 (100%),
92 (54%)
IR (neat): 3205 (br), 1675 (s), 1307 (s), 1253 (s), 1220 (s), and
757 (s) cm
1
1
H NMR (CDCl
3
, 300 MHz): δ 3.92 (s, 3H)
6.85 (t, J 8 Hz, 1H)
7.00 (d, J 8 Hz, 1H)
7.44 (t, J 8 Hz, 1H)
7.83 (d, J 8 Hz, 1H)
10.8 (s, 1H)
13
C NMR (CDCl
3
): δ 52.1 (q), 112.7 (s), 117.7 (d), 119.2 (d),
(hydrogen coupled)
130.1 (d), 135.7 (d), 162.0 (s), 170.7 (s)
PROBLEM 15.87 Dehydration of α-terpineol (A) affords a
mixture of at least two isomers, one of which is B.
Ph
Ph
A
(dimer)
O
B
hν
or Δ
CH
3
H
3
C
AB
O
165.1 84.2
CH
3
OCH
2
CH
H
Isomer B, which exhibits the pleasant odor of lemons and can
be isolated from the essential oils of cardamom, marjoram, and
coriander, reacts with maleic anhydride to yield compound C,
mp 64–65 °C. Spectral data for compound C are summarized
on the next page. Deduce the structures of compounds B and
C. (You may have to make an educated guess about the stereo-
chemistry of compound C.)
Compound C
Mass spectrum: m/z 234 (M)
IR (Nujol®): 1870 (shoulder), 1840 (m), and 1780 (s) cm
1
1
H NMR (CDCl
3
): δ 0.98 (d, J 6.8 Hz, 3H)
1.08 (d, J 6.8 Hz, 3H)
1.36 (m, 4H)
1.46 (s, 3H)
2.60 septet ( J 6.8 Hz, 1H)
2.80 (d, J 9 Hz, 1H)
3.20 (d, J 9 Hz, 1H)
6.01 (d, J 8.5 Hz, 1H)
6.10 (d, J 8.5 Hz, 1H)
PROBLEM 15.88 Isoquinoline (1) undergoes the Chichibabin
amination reaction when treated with potassium amide in liquid
ammonia to yield 1-aminoisoquinoline (2) (p. 676).
15.11 Additional Problems 761
2. H
2
O
1. KNH
2
/NH
3
N
12
N
NH
2
2
1
4
8
5
7
63
PROBLEM 15.89 When reactions are run in THF, it is often
possible to isolate traces of an impurity, compound 1.
Compound 1 gives 81.76% C and 10.98% H upon elemental
analysis. Spectral data for 1 are summarized below. Deduce the
structure of compound 1 and explain your reasoning.
Compound 1
Mass spectrum: m/z 220 (M, 23%), 205 (100%), 57 (27%)
IR (Nujol®): 3660 (m, sharp) cm
1
1
H NMR (CDCl
3
): δ 1.43 (s, 18H), 2.27 (s, 3H), 5.00 (s, 1H,
shifts with D
2
O), 6.98 (s, 2H)
13
C NMR (CDCl
3
): δ 21.2 (q), 30.4 (q), 34.2 (s), 125.5 (d),
128.2 (s), 135.8 (s), 151.5 (s)
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 15.90 Select the reaction “Nucleophilic aromatic
substitution.” Predict the
1
H NMR spectra of the starting
material (1-chloro-2,4-dinitrobenzene) and the product
(N-methyl-2,4-dinitroaniline). How would the IR data
differ for the starting material and the product?
PROBLEM 15.91 View the “Alkene epoxidation” reaction.
Predict the
1
H NMR spectra for the starting material
and the product. Compare the
1
H NMR spectra of the starting
material and the product for the same reaction using
(Z)-2-butene.
PROBLEM 15.92 The animated “Acidic epoxide ring opening”
and “Basic epoxide ring opening” show isomeric products.
What analytical tool (
1
H NMR,
13
C NMR, IR, MS) could
you use to distinguish between the 2-methoxy-1-propanol
and the 1-methoxy-2-propanol products? What specific
differences would you look for and what methods would
you use?
When the reaction mixture containing 1, potassium amide, and
liquid ammonia was examined at 10 °C by
1
H NMR spec-
troscopy, the following signals were observed (solvent hydrogens
and NH
2
hydrogens not reported):
δ 4.87 (d, J 5.5 Hz, 1H), 5.34 (t, J 7.0 Hz, 1H; signal
collapses to a singlet when amide ion is present in excess),
6.35–7.3 (br m, 5H). Rationalize this low-temperature
1
H NMR spectrum. Hint: For reference sake, the
1
H NMR
spectrum of isoquinoline itself (1) is summarized below:
δ 7.3–8.2 (m, H
4
,H
5
,H
6
,H
7
,H
8
), 8.61 (d, J 6 Hz, H
3
),
9.33 (s, H
1
).

Carbonyl Chemistry 1:
Addition Reactions
762
16.1 Preview
16.2 Structure of the
Carbon–Oxygen Double Bond
16.3 Nomenclature of Carbonyl
Compounds
16.4 Physical Properties of
Carbonyl Compounds
16.5 Spectroscopy of Carbonyl
Compounds
16.6 Reactions of Carbonyl
Compounds: Simple Reversible
Additions
16.7 Equilibrium in Addition
Reactions
16.8 Other Addition Reactions:
Additions of Cyanide and
Bisulfite
16.9 Addition Reactions Followed
by Water Loss: Acetal
Formation
16.10 Protecting Groups in Synthesis
16.11 Addition Reactions of Nitrogen
Bases: Imine and Enamine
Formation
16.12 Organometallic Reagents
16.13 Irreversible Addition
Reactions: A General Synthesis
of Alcohols
16.14 Oxidation of Alcohols to
Carbonyl Compounds
16.15 Retrosynthetic Alcohol
Synthesis
16.16 Oxidation of Thiols and Other
Sulfur Compounds
16.17 The Wittig Reaction
16.18 Special Topic: Biological
Oxidation
16.19 Summary
16.20 Additional Problems
16
RETINAL CRYSTAL This is a polarized light photo of retinal (vitamin A), which is
a nutritionally important aldehyde found in green vegetables and fruit (see p. 533).
16.1 Preview 763
“You have two choices,” Jamf cried, his last lecture of the year: outside were
the flowery strokings of wind, girls in pale colored dresses, oceans of beer,
male choruses intensely, movingly lifted as they sang
Semper sit in flores!
Semper sit in flo-ho-res ...“stay behind with carbon and hydrogen, take
your lunch-bucket in to the works every morning with the faceless droves
who can’t wait to get in out of the sunlight—or move
beyond
. Silicon, boron,
phosphorus—these can replace carbon, and can bond to nitrogen instead of
hydrogen.—” a few snickers here, not unanticipated by the playful old
pedagogue, be he always in flower: his involvement in getting Weimar to
subsidize IG’s Stickstoff Syndikat was well known—“move beyond life,
toward the inorganic. Here is no frailty, no mortality—here is Strength, and
the Timeless.” Then his well-known finale, as he wiped away the scrawled
C—H on his chalkboard and wrote, in enormous letters, Si—N.
—THOMAS PYNCHON,
1
GRAVITY’S RAINBOW
16.1 Preview
In Chapters 3, 9, and 10 we examined the structure of alkenes and the addition reac-
tions of their carbon–carbon double bonds. Indeed, we have been exploring various
aspects of carbon–carbon double-bond chemistry since Chapter 9. Now it is time to
extend what we know to the chemistry of carbon–oxygen double bonds,the carbonyl
group. Although it bears many similarities to carbon–carbon double bonds, the car-
bon–oxygen double bond is not “just another double bond,” because there are many
important differences.The chemistry of the carbonyl group is the cornerstone of syn-
thetic chemistry. Much of our ability to make molecules depends on manipulation
of carbonyl groups because they allow us to make carbon–carbon bonds. There are
several centers of reactivity in a molecule containing a carbon–oxygen double bond,
and this versatility contributes to the widespread use of carbonyl compounds in syn-
thesis. There is the π bond itself (don’t forget the antibonding π* orbital), and the
nonbonding electrons on oxygen.The centerpiece reaction of this chapter is the acid-
or base-induced addition to the carbon–oxygen double bond. In addition, there is spe-
cial reactivity associated with the hydrogens on the carbon in the position adjacent
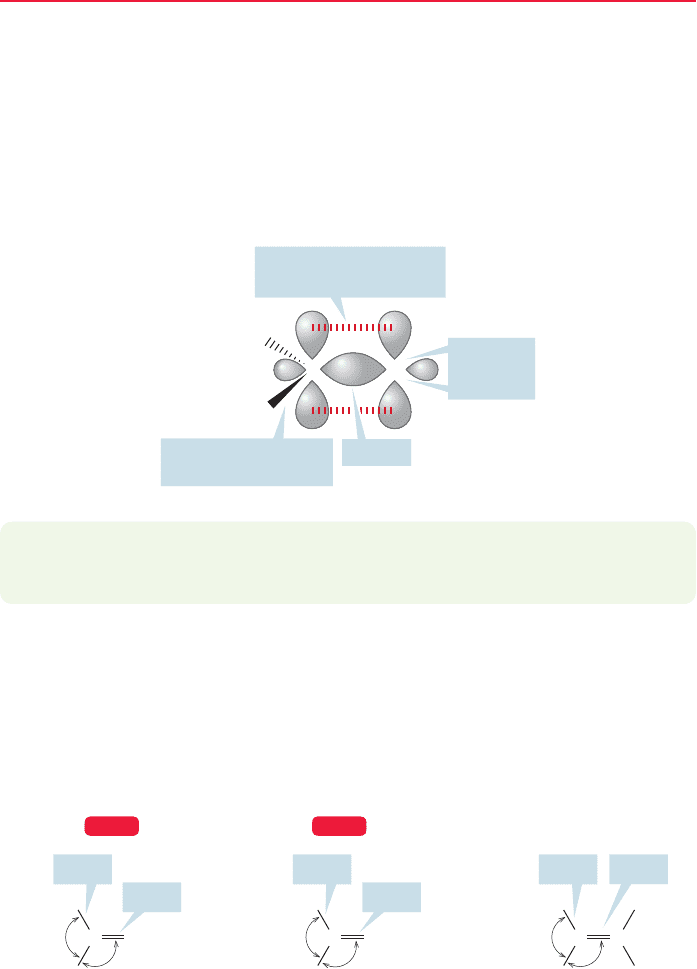
(α) to the carbon–oxygen double bond (Fig. 16.1). These hydrogens are called α
hydrogens. A ketone can have α hydrogens on either side of its carbonyl group. The
chemistry of the α position is very important, but will wait until Chapter 19.
ESSENTIAL SKILLS AND DETAILS
1. Almost this entire chapter involves additions of nucleophiles to carbon–oxygen double
bonds. Many of these reactions are reversible, others are not. It is the reversible
reactions that trouble students most, because there seems to be a bewildering array of
additions, proton transfers, and eliminations. So there is, but keep your wits about you,
and you’ll learn to push those protons and waters around properly.
2. In this chapter, you will learn a lot about synthesis, especially alcohol synthesis. It is not hard
to do these syntheses, but you have to learn the basic reaction—the irreversible addition of
organometallic reagents and metal hydrides to carbonyl compounds—very well.
1
Our first Pynchon quote was in Chapter 9. Same man, same book.
O
..
..
An α hydrogen
π Bond
C
C
R
H
Nonbonding
electrons
FIGURE 16.1 A generic carbonyl
group has three loci of reactivity: the
nonbonding electrons on the oxygen,
the π bond, and its α hydrogens.
764 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
3. A compound does not have to be favored at equilibrium to be the reactive ingredient
in a chemical reaction. Even if it is disfavored, it can still have low barriers to further
reaction. In such a case, it is used up, then regenerated at equilibrium. In such a way, all
of the product can come from the minor partner in an equilibrium.
4. It is very useful to become comfortable with the redox interconversion of carbonyl
compounds (aldehydes and ketones) and the related alcohols.There is a lot of detail
here, and a none-too-simple mechanism as well.
5. Protecting groups are introduced in this chapter. A reactive functional group can be
converted into a nonreactive group and stored in this fashion. Later, when the reactivity
of the functional group is required, it can be regenerated as needed.There are many
protecting groups, and it is very helpful to be able to use them in synthesis.
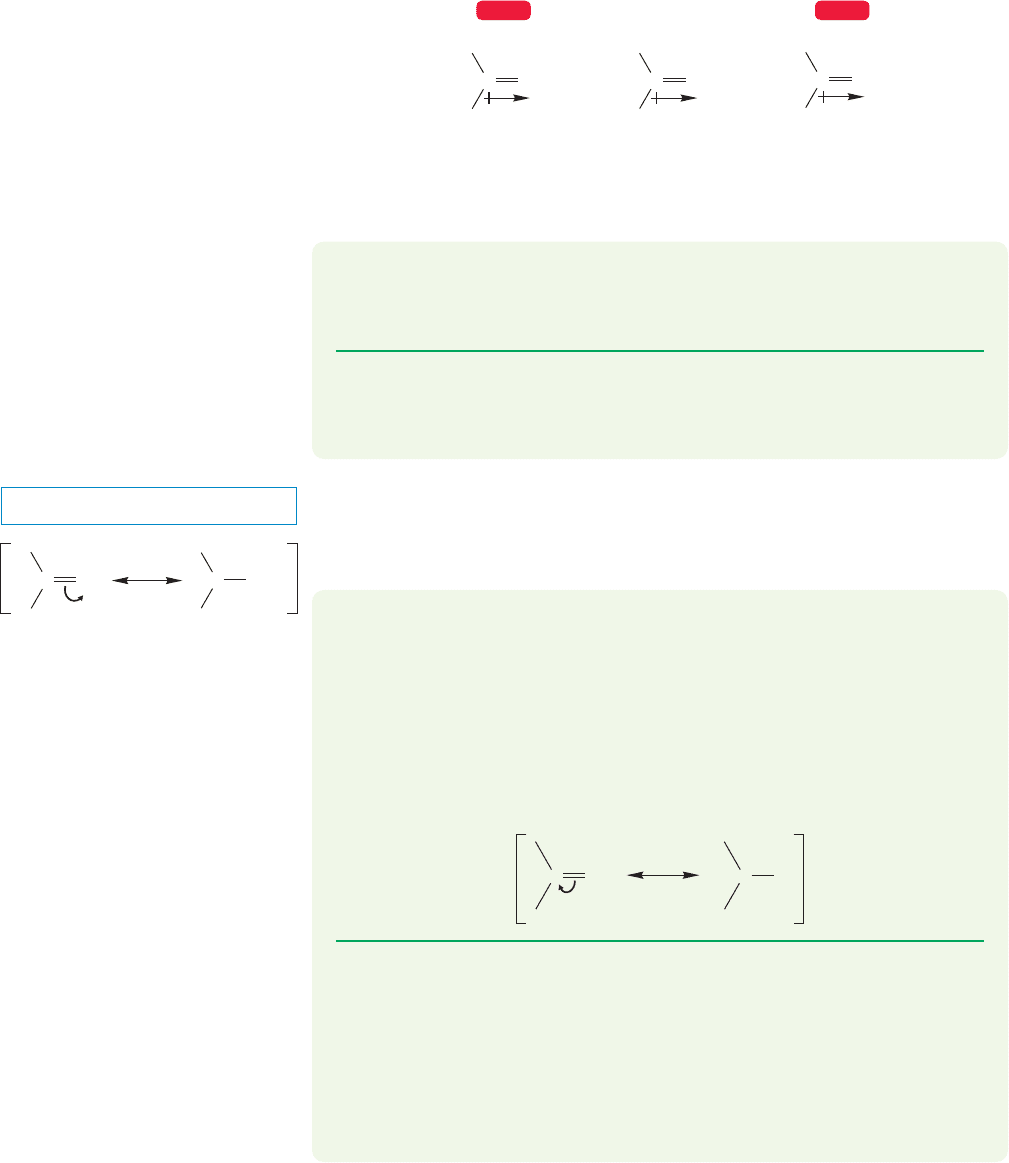
16.2 Structure of the Carbon–Oxygen Double Bond
By analogy to the carbon–carbon double bond, we might expect sp
2
hybridization
for the carbon atom in the carbonyl group. The 2p orbitals on carbon and oxygen
form the π bond,and two lone pairs of electrons remain on oxygen.Figure 16.2 gives
a schematic orbital picture of both the σ and π systems for the simplest carbonyl
compound, formaldehyde.
..
..
H
H
C
O
C9 H Bond made
from 1s /sp
2
overlap
π Bond made from two
overlapping 2p orbitals
σ Bond
Lone-pair
electrons
FIGURE 16.2 One orbital picture of
the simplest carbonyl compound,
formaldehyde.
The structures of some known carbonyl compounds show that this picture is a
reasonable one. Bond angles are close to the 120° required by pure sp
2
hybridiza-
tion,and of course we would not expect a molecule of less than perfect trigonal sym-
metry to have exactly sp
2
hybridization. Bond lengths are similar to those in simple
alkenes, although the carbon–oxygen double bond is a bit shorter and stronger than
its carbon–carbon counterpart (Fig. 16.3).
PROBLEM 16.1 Make an orbital interaction diagram for each different kind of
bond in the molecule formaldehyde, shown in Figure 16.2.
Methanal
(formaldehyde)
Ethanal
(acetaldehyde)
Ethene
(ethylene)
118⬚
121⬚
1.06
A
⬚
1.23 A
⬚
CO
H
H
118⬚
121⬚
1.09
A
⬚
1.22 A
⬚
CO
H
C
H
H
H
3
C
116.6⬚
121.7⬚
1.08
A
⬚
1.33 A
⬚
C
H
H
WEB 3D WEB 3D
FIGURE 16.3 A comparison of the
structures of formaldehyde,
acetaldehyde, and ethylene.

16.2 Structure of the Carbon–Oxygen Double Bond 765
Formaldehyde is the smallest and
simplest aldehyde. It can be found
in substantial concentrations in new
buildings as commonly used wood
products age.
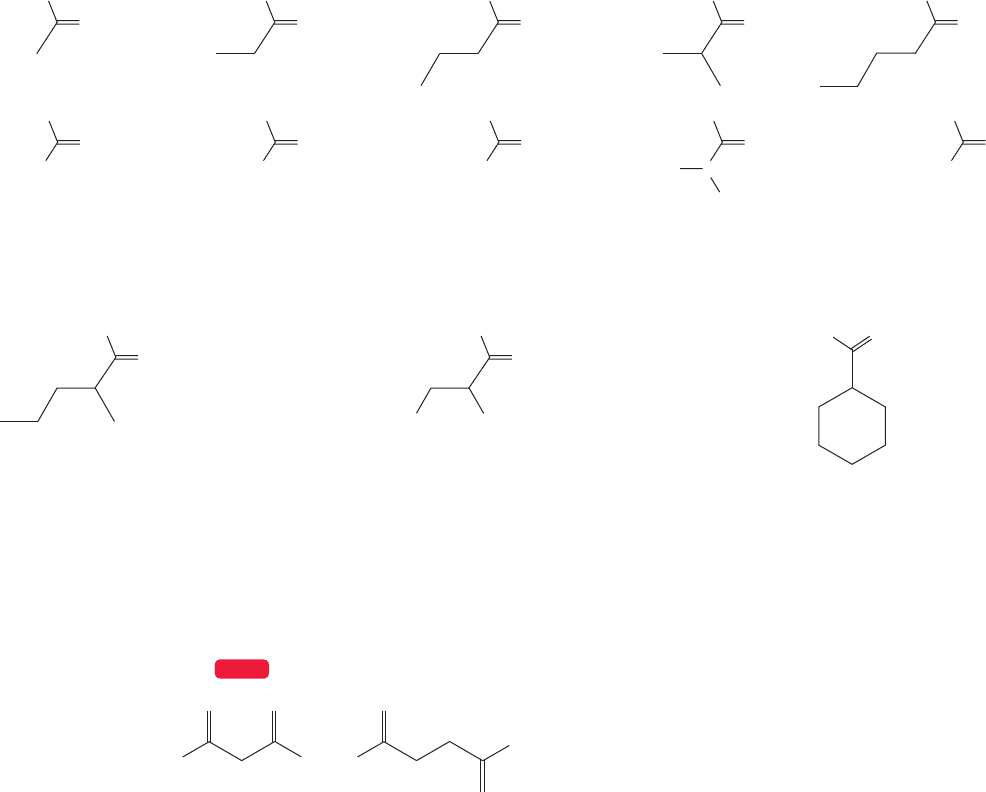
The carbonyl π bond is constructed in the same way as the related carbon–
carbon π bond.The 2p orbitals on the adjacent carbon and oxygen atoms are allowed
to interact in a constructive way to form a bonding π molecular orbital, and in a
destructive, antibonding way to form π*. But there is a difference. In an alkene, the
constituent 2p orbitals are of equal energy, and therefore contribute equally to the
formation of π and π*. In a carbonyl group, the two 2p orbitals are not identical.
An electron in the 2p orbital on the more electronegative oxygen atom is lower in
energy than one in the 2p orbital on the carbon atom, so the oxygen atom con-
tributes more to π than the more energetically remote carbon 2p orbital (Fig. 16.4).
The bonding π molecular orbital will be constructed from more than 50% of the
oxygen 2p atomic orbital and less than 50% of the carbon 2p atomic orbital. In a
similar way, we can see that π* will be made up of more than 50% of the energeti-
cally close carbon 2p orbital and less than 50% of the energetically more remote
2p orbital on oxygen.
Energy
C
O
C 2p
O 2p
π*
π
C
O
FIGURE 16.4 A graphical
construction of π and π* for the
carbonyl group.The π orbital is
constructed from more than 50% of
the oxygen 2p orbital, and the π*
orbital from more than 50% of the
carbon 2p orbital.
So the carbonyl π and π* orbitals bear a general resemblance to the π and π*
orbitals of the alkenes, but are less symmetrical. The two electrons naturally occu-
py the lower energy bonding molecular orbital. In the π molecular orbital, there is
766 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
FIGURE 16.5 Carbonyl compounds
are polar molecules with substantial
dipole moments [measured in debye
(D) units]. Notice the small bond
dipole arrow that points from the
positive end toward the negative end
of the dipole.
a greater probability of finding an electron in the neighborhood of the relatively elec-
tronegative oxygen atom than near the more electropositive carbon, and this unequal
sharing of electrons contributes to the dipole moment in molecules containing
carbonyl groups. As can be seen from Figure 16.5, carbonyl compounds do have
substantial dipole moments and are quite polar molecules.
PROBLEM 16.2 It is not just the π bonds that are unsymmetrical in a carbonyl
group. Use the hybrid orbitals of oxygen and carbon to construct the C–O σ and
σ* orbitals.
PROBLEM 16.3 One way to construct a carbon–oxygen double bond using the
hybridization formalism is to use sp-hybridized oxygen. Make a drawing of this
model in the style of Figure 16.2.
In resonance terms, we can describe the carbonyl group as a combination of
the two major contributing forms shown in Figure 16.6.This picture emphasizes the
Lewis acid (electrophilic) character of the carbon of the carbon–oxygen double bond.
C CO
..
..
+
–
O
..
..
..
A GENERAL CARBONYL GROUP
FIGURE 16.6 A resonance
formulation of a carbonyl group.
+
–
C
O
..
..
C
O
..
..
..
WORKED PROBLEM 16.5 Why is the dipole moment in acetone larger than that in
acetaldehyde (Fig. 16.5)?
ANSWER Because the negative end of the dipole is the same for each species,
there can be no answer there. However, the two methyl groups in acetone stabi-
lize the positive charge much better than the methyl and hydrogen of acetalde-
hyde do. The polar form is more stable in acetone than it is in acetaldehyde, and
as a result, acetone has a larger dipole moment.
H
H
2.33 D
2.88 D
2.69 D
C
H
H
3
C
C
H
3
C
H
3
C
O
..
..
O
..
..
δ
+
δ
–
δ
+
δ
–
CO
..
..
δ
+
δ
–
Methanal
(formaldehyde)
Ethanal
(acetaldehyde)
Propanone
(acetone)
WEB 3D WEB 3D
WORKED PROBLEM 16.4 There is another possible polar resonance form in addi-
tion to the one shown in Figure 16.6. What is it, and why is it not considered a
major contributor to the carbonyl group?
ANSWER The other polar resonance form has the positive charge on oxygen and
the negative charge on carbon. Oxygen is more electronegative than carbon and
the negative charge will be more stable on oxygen than on carbon. Similarly, the
relatively electropositive carbon should have the positive charge, not the negative.
For these reasons, this form is not a major contributor to the structure.
16.3 Nomenclature of Carbonyl Compounds 767
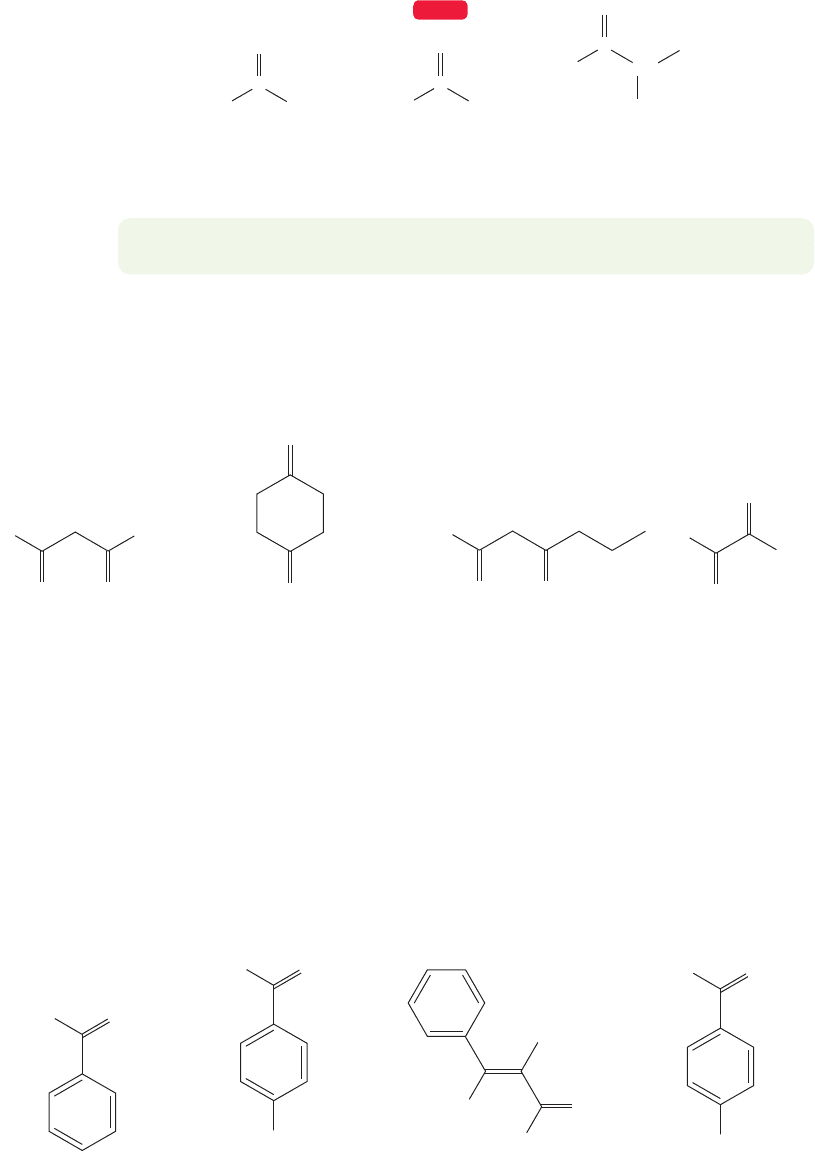
16.3 Nomenclature of Carbonyl Compounds
In a generic carbonyl compound R
2
C O, there is a vast array of possible substi-
tution patterns,and there is a separate name for each one of them. Simple compounds
in which both R groups are hydrocarbons such as alkyl, alkenyl, or aryl are called
ketones. If one R group is hydrogen, the compound is an aldehyde, and the mol-
ecule in which both R groups are hydrogen is called formaldehyde.
Common names are often used for the aldehydes containing four carbons or fewer,
but thereafter the IUPAC system takes over, and the naming process becomes ration-
al. The “e” of the parent alkane’s name is dropped and the suffix “al” is added. In the
IUPAC numbering system the aldehyde group is always number 1. When aldehydes
are attached to cycloalkanes, the term carboxaldehyde is used,as in “cyclopropanecar-
boxaldehyde.” Some aldehydes and their IUPAC names are given in Figure 16.7.
P
Cyclohexanecarboxaldehyde
HO
3-Chloro-2-hydroxypropanal
H
Cl OH
2-Methylpentanal
H
O
Pentanal
CH
3
CH
2
CH
2
CH
2
H
H
Butanal
(butyraldehyde)
H
CH
3
CH
2
CH
2
H
2-Methylpropanal
(isobutyraldehyde)
H
CH
3
CH
3
CH
H
H
CH
3
CH
2
H
Propanal
(propionaldehyde)
H
O
H
3
C
H
Ethanal
(acetaldehyde)
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
..
..
O
..
..
..
..
FIGURE 16.7 A few aldehydes and their IUPAC names are shown. Common names are given in parentheses.
Compounds containing two aldehydes are dials. In this case, the final “e” of the
parent name is not dropped before adding “dial” (Fig. 16.8).
Propanedial
O
..
..
O
..
..
H
H
Butanedial
O
..
..
H
H
O
..
..
WEB 3D
FIGURE 16.8 Dialdehydes are named
as dials.
Ketones, too, have common names, but most have been discarded. An exception
is the name for the simplest member of the class, dimethyl ketone,which is still called
acetone. Ketones are named in several ways. For example, acetone is also known as
768 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
O
..
..
Propanone
(acetone)
(dimethyl ketone)
Butanone
(ethyl methyl ketone)
3-Methyl-2-pentanone
(sec-butyl methyl ketone)
C
CH
3
H
3
C
O
..
..
C
CH
3
CH
3
CH
2
O
..
..
C
CH
CH
3
H
3
C
CH
2
CH
3
WEB 3D
FIGURE 16.9 Simple ketones can be
named in many ways. The IUPAC
names are listed first.
PROBLEM 16.6 Why is acetone properly named propanone and not 2-propanone?
The rules are fairly simple: in the presence of a higher priority group the IUPAC
system treats the carbonyl oxygen atom as a substituent that is described by the
prefix “oxo-” and given a number like any other attached group. A great many
common names are retained for aromatic aldehydes and ketones. For example, the
simplest aromatic aldehyde is not generally called “benzenecarboxaldehyde” but
benzaldehyde. That one’s quite easy to figure out, but most are not. A few com-
monly used names are given in Figure 16.11.
Compounds containing two ketones are diones. As with dials, the final “e” of
the parent alkane is retained,and the name begins with the position numbers of the
two carbonyl groups (Fig. 16.10).Ketoaldehydes are named as aldehydes, not ketones,
because the aldehyde group has a higher priority than a keto group (Fig. 16.10).
2-oxopropane, propanone, or dimethyl ketone (Fig. 16.9). In using the IUPAC sys-
tem, the final “e” of the parent alkane is dropped, and the suffix “one” is appended.
The number of the carbon atom bearing the carbon–oxygen double bond is placed
before the parent name.
2,4-Pentanedione
1,4-Cyclohexanedione
O
..
..
H
O
..
..
O
..
..
O
..
..
O
..
..
CH
3
CH
3
H
3
C
3-Oxohexanal
2-Oxopropanal
(pyruvic aldehyde)
O
..
..
O
..
..
O
..
..
H
FIGURE 16.10 Diketones are named as diones. The final “e” of the parent hydrocarbon is retained.
Ketoaldehydes are named as aldehydes, not ketones.
O
..
O
..
..
..
..
..
..
OCH
3
H
H
H
O
..
..
CH
3
H
O
..
H
Benzaldehyde
4-Methoxybenzaldehyde
( p-anisaldehyde)
4-Methylbenzaldehyde
(p-tolualdehyde)
(E)-3-Phenyl-2-propenal
(trans-cinnamaldehyde)
H
FIGURE 16.11 Some common names
for aromatic aldehydes are shown.
The IUPAC accepted names
are listed first.
