Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

16.3 Nomenclature of Carbonyl Compounds 769
CIVETONE
This simple, but unusual cycloalkenone is called civetone,
as it is isolated from a gland present in both male and
female civet cats, Viverra civetta and Viverra zibetha. The
cats use it as a sexual attractant and marking agent. It has a
strong musky odor, overwhelming when concentrated (to
humans, that is, probably not to civet cats). In high dilution
the odor becomes quite pleasant, and civetone and the
related cyclic 15-membered ring ketone, muskone, are
important starting materials in the perfumery industry.
In the old days, you had to catch yourself a mess o’ civet
cats, described as “savage and unreliable,” to make your
perfumes. Nowadays, this compound can be made quite
easily in the laboratory, a fact presumably reassuring to the
civet cat population.
Civet
Civetone
H
2
C
H
2
C
H
2
C
H
2
C
C
C
C
O
H
H
H
2
C
H
2
C
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
Acetophenone
(methyl phenyl ketone)
2-Methyl-1-phenyl-1-propanone
(isopropyl phenyl ketone)
Benzophenone
(diphenyl ketone)
O
..
..
O
..
..
O
..
..
H
3
C
WEB 3D
FIGURE 16.12 Ketones containing
benzene rings are phenyl ketones.
This figure also shows the “ophenone”
method of naming these molecules.
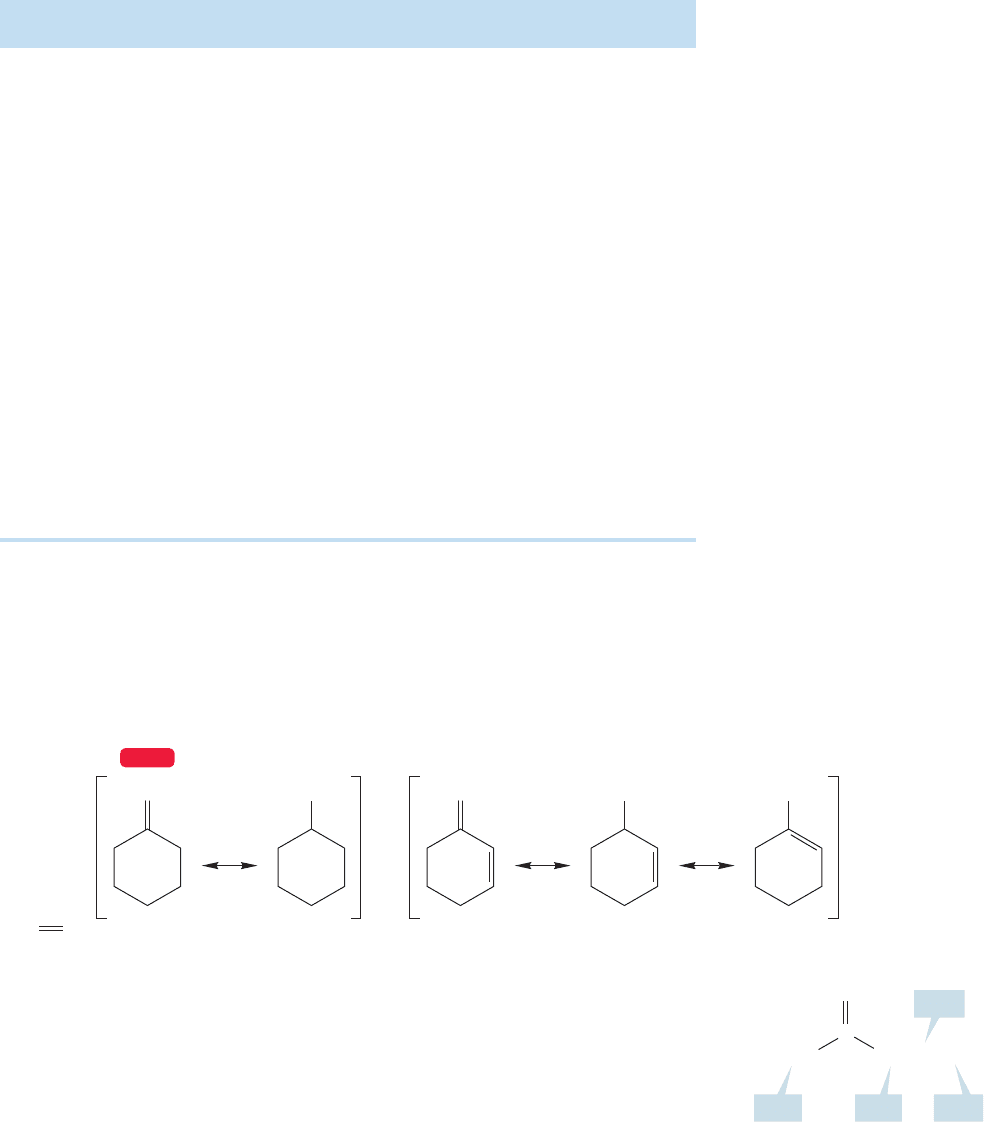
Aromatic ketones are often named by treating the benzene ring as a phenyl group,
as in isopropyl phenyl ketone (Fig. 16.12). A few can be named through the IUPAC
system in which the framework Ph—C O is indicated by the term “ophenone” and
the remainder of the molecule designated by one of the alternative names in Figure 16.12.
Thus, diphenyl ketone is benzophenone, methyl phenyl ketone is acetophenone, ethyl
phenyl ketone is propiophenone,and phenyl propyl ketone is butyrophenone (Fig.16.12).
P
C
R
Carboxylic acid Acid anhydrideAcid chlorideEster Amide
O
..
..
C
R
O
..
..
C
R
O
..
..
C
R Cl
O
..
..
..
..
..
C
R
O
..
..
NH
2
..
OR
..
..
C
O
..
..
RO
..
..
OH
..
..
WEB 3D WEB 3D
FIGURE 16.13 Some names for
other carbonyl compounds in
which one R group is neither
hydrogen nor a hydrocarbon
group.
Figure 16.13 gives the names of some compounds containing carbon–oxygen dou-
ble bonds in which one of the R groups is neither hydrogen nor a hydrocarbon group.
We met these compounds in earlier chapters and we will see them again in later
chapters and deal with their nomenclature more extensively there.

770 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
16.4 Physical Properties of Carbonyl
Compounds
Carbonyl compounds are all somewhat polar, and the smaller, less hydrocarbon-like
molecules are all at least somewhat water soluble. Acetone and acetaldehyde are
miscible with water, for example. Their polarity means that they can profit
energetically by associating in solution, and therefore their boiling points are
considerably higher than those of hydrocarbons containing the same number of
carbons and oxygens. For example, propane and propene are gases with boiling
points of 42.1 °C and 47.4 °C, respectively. By contrast, acetaldehyde boils at
20.8 °C, about 63 degrees higher. Acetone boils more than 60 degrees higher
than 2-methylpropene (isobutene).
Table 16.1 gives some physical properties of a number of common carbonyl-
containing compounds (and some related hydrocarbons in parentheses).
TABLE 16.1 Some Physical Properties of Carbonyl Compounds
Compound bp (°C) mp (°C) Density (g/mL) Dipole Moment (D)
Formaldehyde 21 92 0.815 2.33
Acetaldehyde 20.8 121 0.783 2.69
Propionaldehyde 48.8 81 0.806 2.52
(Propane)
a
42.1
(Propene)
a
47.4
Benzaldehyde 178.6 26 1.04
Acetone 56.2 95.4 0.789 2.88
(Isobutene)
a
6.9
Ethyl methyl ketone 79.6 86.3 0.805
Cyclohexanone 155.6 16.4 0.948
Methyl phenyl ketone 202.6 20.5 1.03
Diphenyl ketone 305.9 48.1 1.15 2.98
a
Hydrocarbons for comparison purposes.
16.5 Spectroscopy of Carbonyl Compounds
16.5a Infrared Spectroscopy Carbonyl compounds are ubiquitous in
nature. The C O bond is one of the most widely found of the “functional
groups.” Spectroscopy, especially IR spectroscopy, played an important role in
identifying many medicinally and otherwise important compounds.The carbon–
oxygen double bond has a diagnostically useful and quite strong stretching fre-
quency in the IR, generally near 1700 cm
1
(p. 711). This absorption is at
substantially higher frequency than that of carbon–carbon double bonds.
This shift should be no surprise, because we already know that carbon–oxygen
double bonds are stronger than carbon–carbon double bonds, and therefore
require more energy for stretching. A useful diagnostic for aldehydes is the
pair of bands observed in the C—H stretching region at about 2850 cm
1
and
2750 cm
1
. Table 16.2 gives some IR, NMR, and UV data for a few aldehydes
and ketones.
P
16.5 Spectroscopy of Carbonyl Compounds 771
TABLE 16.2 Some Spectral Properties of Carbonyl Compounds
Compound IR
13
C NMR UV
(CCl
4,
cm
1
)(δ C O) [nm (ε)]
Formaldehyde 1744 (gas phase) 270
Acetaldehyde 1733 199.6 293.4 (11.8)
Propanal 1738 201.8 289.5 (18.2)
Acetone 1719 205.1 279 (14.8)
Cyclohexanone 1718 207.9 285 (14)
Cyclopentanone 1751 213.9 299 (20)
Cyclobutanone 1788 208.2 280 (18)
Butanone 1723 206.3 277 (19.4)
3-Butenone 1684 197.2 219 (3.6)
2-Cyclohexenone 1691 197.1 224.5 (10,300) (ππ*)
Benzaldehyde 1710 191.0 244 (15,000) (ππ*)
328 (20) (n π*)
Benzophenone 1666 195.2 252 (20,000) (ππ*)
325 (180) (n π*)
Acetophenone 1692 196.8 240 (13,000) (ππ*)
319 (50) (n π*)
U
U
U
U
U
U
U
P
Note that conjugated carbonyl compounds are always found at lower frequency
than their unconjugated relatives.For example,cyclohexanone appears at 1718 cm
1
and 2-cyclohexenone at 1691 cm
1
(Fig. 16.14).Thus there is reduced double-bond
character in the C O group in conjugated molecules.The resonance formulation
of 2-cyclohexenone clearly shows the partial single-bond character of the carbon–
oxygen bond in these compounds.
P
stretching
frequency
Cyclohexanone 2-Cyclohexenone
1718 cm
–1
1691 cm
–1
IR C
O
+
–
+
WEB 3D
O
O
O
O
–
+
O
O
O
O
–
O
O
FIGURE 16.14 Conjugation reduces the double-bond character of a carbonyl
group. The result is a shift of the IR stretching band to lower frequency.
C
CH
2
CH
2
CH
3
O
H
3
C
δ 2.1 δ 2.4
δ 1.6
δ 0.9
FIGURE 16.15 Hydrogens on the
α carbons are affected most by the
electron-withdrawing carbonyl
group. Hydrogens on the β carbon
are only slightly shifted. Hydrogens
that are further away are not
significantly affected (δ in ppm).
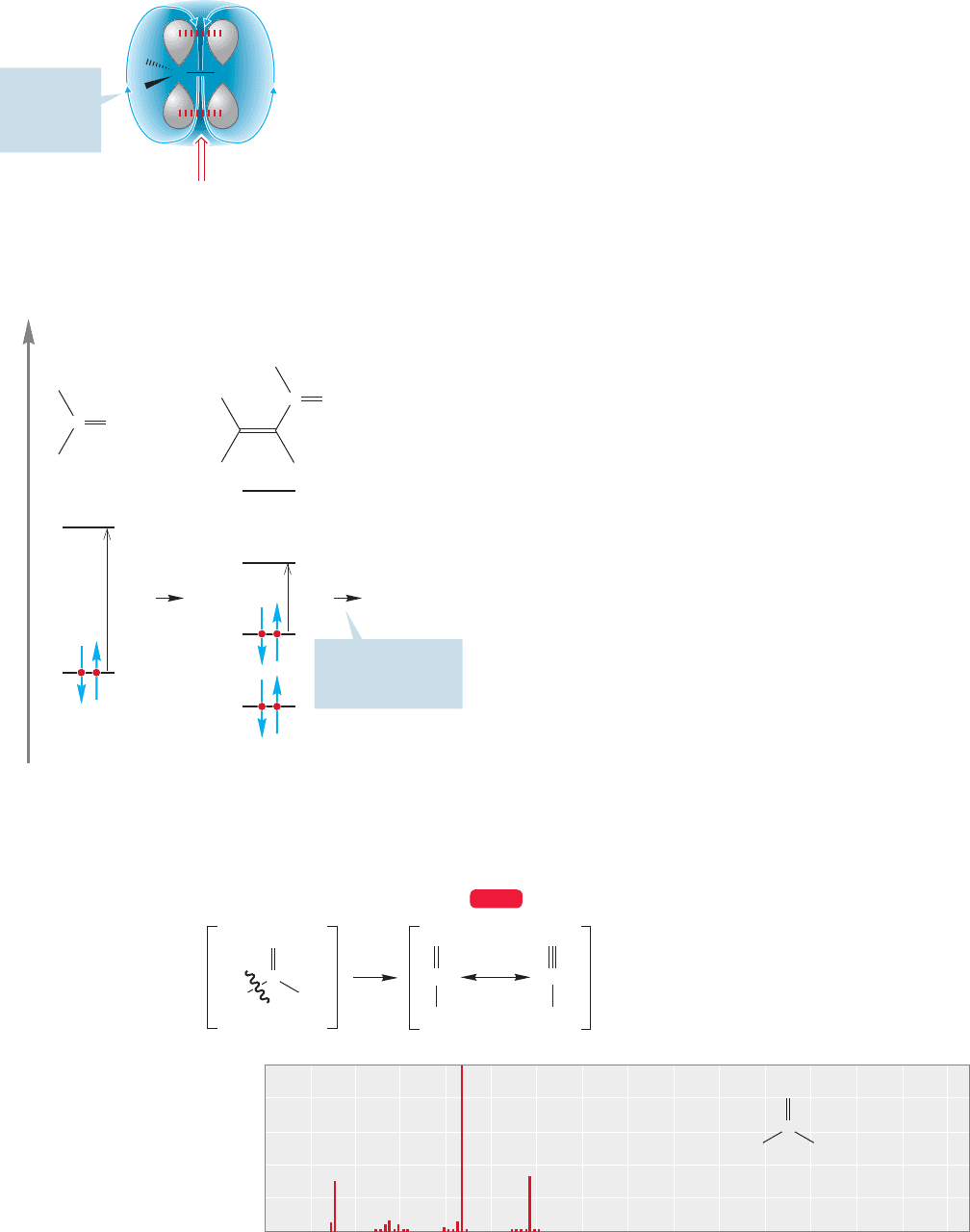
16.5b Nuclear Magnetic Resonance Spectroscopy The carbonyl group
inductively withdraws electrons from the nearby positions, and this inductive effect
results in a deshielding of adjacent hydrogens.The further away from the carbonyl
group, the less effective the deshielding.This effect is well illustrated by 2-pentanone
(Fig. 16.15).
In aldehydes, hydrogens directly attached to the carbonyl group resonate far
downfield,δ 8.5–10 ppm. Not only does the carbon bear a partial positive charge
that deshields the attached hydrogen, but the applied magnetic field B
0
creates an
induced field B
i
in the carbonyl group that augments the applied field in the region
'
772 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
..
..
R
H
C
O
At this point
B
0
and B
i
reinforce
each other
B
i
B
i
B
0
FIGURE 16.16 Aldehyde hydrogens
are in a strongly deshielded region in
which the induced magnetic field B
i
augments the applied field B
0
.
of aldehydic hydrogens (Fig. 16.16). Accordingly, a relatively weak applied magnetic
field is required to reach the resonance frequency, and they appear unusually far
downfield (p. 720).
The carbon atoms of carbonyl groups carry partial positive charges and are
greatly deshielded by their relative lack of surrounding electrons. They, too, res-
onate especially far downfield in the
13
C NMR. Table 16.2 shows typical reso-
nance positions.
16.5c Ultraviolet Spectroscopy Simple aldehydes and ketones, like
simple alkenes, do not have a ππ* absorption in the region of the UV spec-
trum accessible to most spectrometers. However, unlike alkenes, carbonyl com-
pounds have another possible absorption which, though weak, is detectable.
This absorption involves promotion of a nonbonding, or
n, electron to the antibonding π* orbital, the n π*
absorption. Table 16.2 gives wavelengths for UV absorp-
tions of a few compounds.
As for alkenes (p. 528), conjugation of the carbonyl group
greatly reduces the energy necessary to promote an electron
from the highest occupied π molecular orbital to the
LUMO. So, the strong ππ* transition in unsaturated
carbonyl compounds can be detected because of the conju-
gation-induced shift to the longer wavelengths (lower ener-
gy) observable by normal UV/vis spectrometers (Table 16.2;
Fig. 16.17).
16.5d Mass Spectrometry There is a diagnostic cleav-
age reaction that occurs for many simple carbonyl-containing
compounds. It involves breaking the bond attached directly to
the carbonyl carbon, with the charge remaining on the car-
bonyl group. Cleavage of this bond generates a resonance-
stabilized acylium ion. It is this resonance stabilization that
accounts for the prominence of this particular fragmentation.
Figure 16.18 shows the mass spectrum for acetone, the arche-
typal methyl ketone.
U
U
U
C
O
..
..
π*
π
LUMO
h
ν (π π*)
LUMO
O
..
..
C
This lower energy
absorption can be
observed
Energy
hν (π π*)
FIGURE 16.17 Conjugation leads to lower energy (longer
wavelength) π→π* absorptions that are observable by UV/vis
spectrometers.
H
3
C
O
CH
3
CH
3
C
+
+
+
m/z
80
0
20
40
60
80
100
Relative Abundance
100 110 120 130 140 1502010 30 40 50 60 70 90
m/z = 43
The acylium ion
O
C
CH
3
O
C
H
3
C
M = 68
O
CH
3
C
.
+
CH
3
.
WEB 3D
FIGURE 16.18 In the mass
spectrum of acetone, the
base peak results from
formation of the
resonance-stabilized
acylium ion.
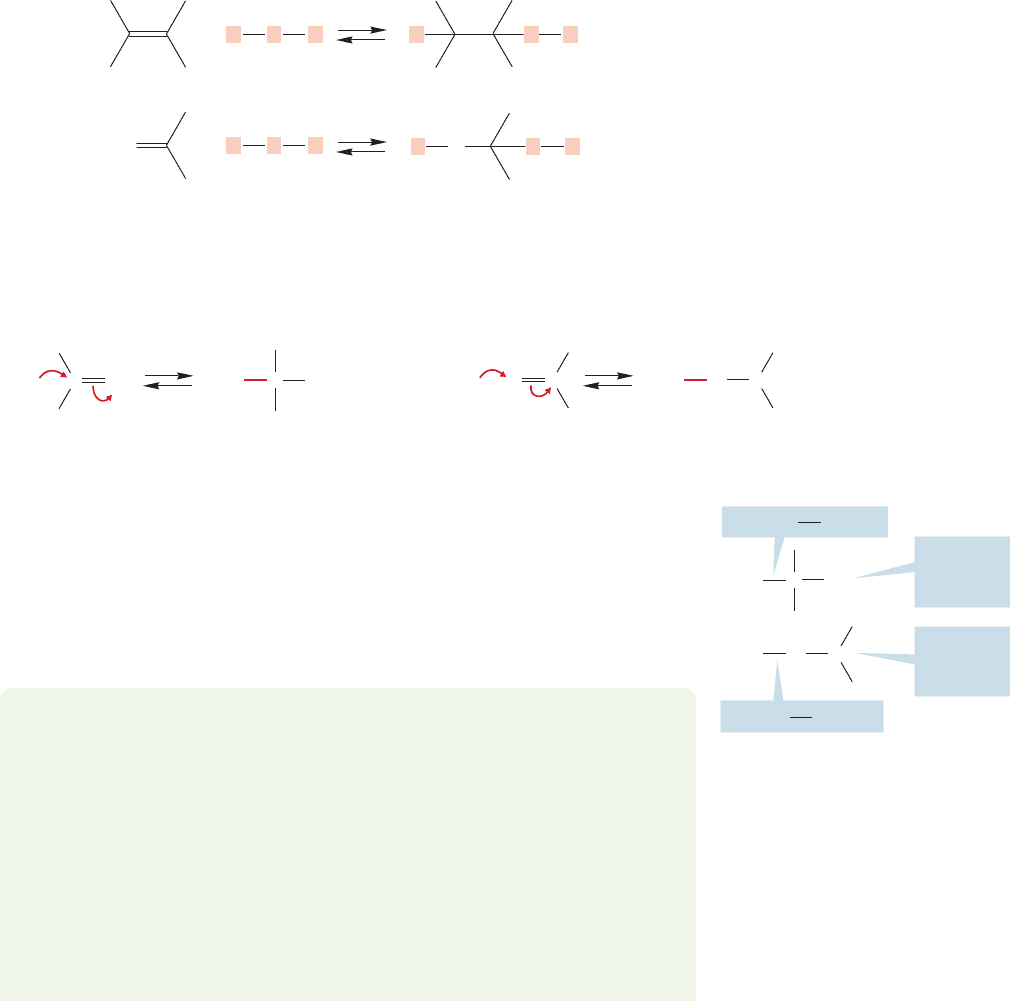
16.6 Reactions of Carbonyl Compounds: Simple Reversible Additions 773
16.6 Reactions of Carbonyl Compounds: Simple
Reversible Additions
In simple addition reactions, carbonyl compounds can behave as either Lewis acids
or Lewis bases depending on the other reagents present. As we might expect, there
is some correspondence between addition reactions of carbon–oxygen double bonds
and those of the carbon–carbon double bonds we saw in Chapters 9 and 10. As we
might also expect, additional complexity is introduced by the presence of two
different atoms in the carbon–oxygen double bond. Figure 16.19 compares the
reaction between water and a generic carbonyl compound (the formation of a
hydrate) with the hydration of an alkene.
Three factors must be considered in predicting which way the addition will go.
First of all, addition to the carbon of the carbon–oxygen double bond places the neg-
ative charge on the highly electronegative oxygen atom, whereas addition at oxygen
generates a much less stable carbanion (Fig. 16.21).Second,addition to oxygen forms
a weak oxygen–oxygen bond, whereas addition to the carbon makes a stronger
oxygen–carbon bond.
Alkene Alcohol
Carbonyl Hydrate
+
OHH H
..
..
+
OHH
..
..
..
..
H
O
H
..
..
H
O
..
..
O
..
..
O
FIGURE 16.19 Addition of water to
an alkene to give an alcohol is
analogous to addition to a ketone or
aldehyde to give a hydrate.
If water acts as a nucleophile and adds to the Lewis acid carbonyl group, there
is a choice to be made. In principle, water could add to either the carbon or the
oxygen of the carbonyl group (Fig. 16.20).
–
–
C C
O
..
..
..
..
..
O
..
..
H
2
Oor
+
O
..
..
..
H
2
O
+
..
..
H
2
O
..
..
O
C C
..
..
H
2
O
FIGURE 16.20 In principle, addition of water to a carbonyl group could occur in two ways.
–
–
C
O
..
.. ..
..
..
H
2
O
+
O
..
.. ..
H
2
O
+
C
Weak O O bond
Strong O C bond
Negative
charge
on oxygen
Negative
charge
on carbon
FIGURE 16.21 Addition to the
carbon end, which puts the negative
charge on the relatively
electronegative oxygen atom, will be
greatly preferred.
WORKED PROBLEM 16.7 Explain how the thermodynamic preference for placing
a negative charge on oxygen, rather than carbon, can influence the rates of addi-
tion to the two ends of the carbon–oxygen double bond.
ANSWER The rates of reaction depend, of course, on the heights of the tran-
sition states, not on the energies of the products in which a full nega-
tive charge resides on either oxygen or carbon. In the transition states for
addition, there will be a partial negative charge on either oxygen or carbon.
The factors that favor a full negative charge on oxygen rather than carbon
(electronegativity) will also favor a partial negative charge (δ
) on oxygen.
(continued)
774 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
Therefore, in the transition state, a partial negative charge will be more stable
on oxygen than on carbon.
Transition state
C
δ
–
O
..
..
OH
2
C
..
..
O
..
OH
2
δ
+
..
–
C
..
..
O
..
OH
2
+
..
..
..
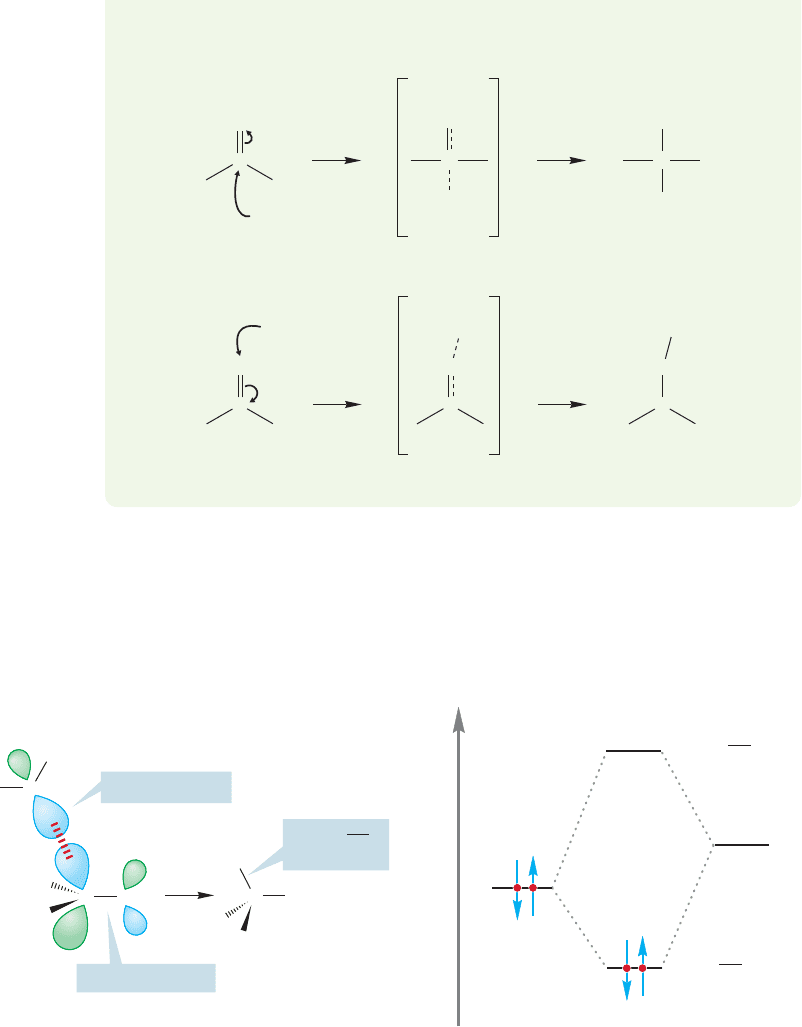
The third factor to consider involves the orbitals making up the carbonyl π
bond. If we look at the molecular orbitals involved, we can see how they influence
the direction in which the reaction occurs. As in all organic reactions, we look for
the combination of a nucleophile (here, water) and an electrophile.The electrophile
in this case must be the empty π* orbital of the carbonyl group. Figure 16.22 shows
the orbital interactions graphically, and gives pictures of the orbital lobes involved.
–
+
O
..
..
..
Empty π* orbital
A filled orbital n
H
H
C
..
C
O
O
H
2
O
π*
σ*
C
O
σ
C
O
n
..
bond
Energy
New O
C
FIGURE 16.22 In the addition of water to a carbonyl group, a filled n orbital of water overlaps with the
empty π* orbital of the carbonyl group. This interaction between a filled and an empty orbital is stabilizing.
As we saw in our earlier section on structure (p. 764), neither the π nor the π*
of the carbonyl group is symmetric with respect to the midpoint of the carbon–
oxygen bond. In particular, the π* is much larger in the region of the carbon atom
than it is near the oxygen. Because the magnitude of stabilization depends on the
amount of orbital overlap, it is no surprise to see that addition at the carbon, where
the π* is largest, dominates the reaction. So, not only is the direction of the addi-
tion affected by the electronegativities and the bond strengths, but it’s affected by
the shapes of the orbitals involved as well.
C
C
δ
–
Transition state
..
..
O
..
..
OH
2
..
..
..
O
..
OH
2
δ
+
C
–
..
..
..
O
..
OH
2
+
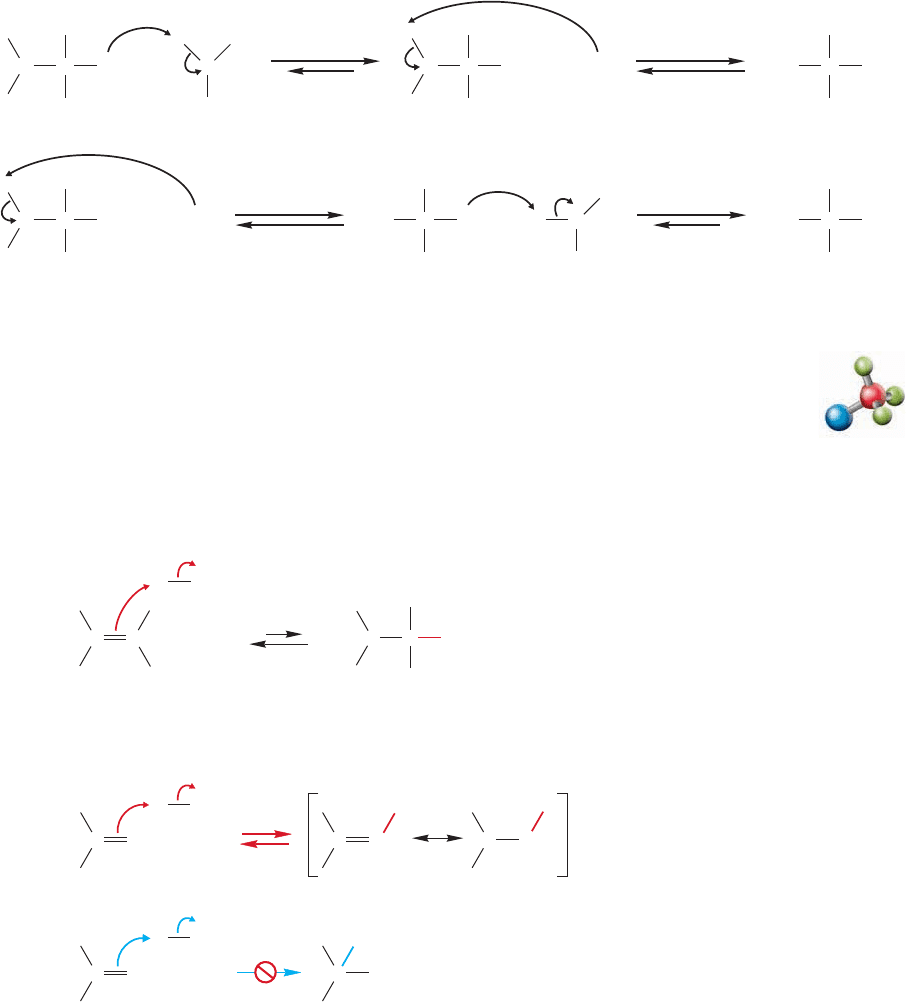
16.6 Reactions of Carbonyl Compounds: Simple Reversible Additions 775
After the addition of water to form the alkoxide, a proton must be removed and
a proton added to give the hydrate, not necessarily in that order (Fig. 16.23). It is
not necessary that the proton lost from one molecule be re-added to the same mol-
ecule.The protonation–deprotonation process is very likely to be intermolecular, as
shown in Figure 16.23.
Now consider how we might make the carbonyl addition reaction easier.Two ways
seem possible—we could make either the electrophile or the nucleophile stronger. Let’s
start with the acid-catalyzed reaction in which the carbonyl group is converted into a
stronger electrophile. If we take as our model for the mechanism the acid-catalyzed
additions to alkenes, a reasonable first step for hydration of a carbonyl would be pro-
tonation. But which end is protonated? Again, there are two possiblities (Fig. 16.24).
protonation
or
OC
..
O
..
..
..
+–
H
2
O
+
deprotonation
H
O
..
+
H
H
H
H
OC
..
OH
..
....
..
+
+
H
H
H
3
O
HO C
..
OH
..
.. ..
..
+
+
deprotonation
OCO
..
..
..
+–
+
protonation
H
O
..
..
+–
H
H
H
HO HC
..
..
O
..
..
..
H
2
O
..
..
+
H
2
O
HO C
..
OH
..
.... ..
..
+
FIGURE 16.23 The carbonyl hydration reaction is completed by two proton transfers. The order of the
two steps is not certain.
C CCHC
H
..
..
OH
2
..
..
Resonance stabilized
Protonation of an alkene (more stable intermediate formed)
Protonation of a carbonyl (two possibilities, more stable intermediate formed)
+
+
+
+
OH
2
..
+
H
OH
2
..
+
+
..
OH
2
+
CO
H
H
..
CO
..
..
..
CO
..
..
H
OH
2
..
+
CO
H
C
..
..
O
FIGURE 16.24 Protonation of the
carbonyl group can occur in two
ways. The placement of a proton on
oxygen to give a resonance-stabilized
cation will be greatly preferred.
The choice is easy this time. First, the carbonyl oxygen atom is a good Brønsted
base because it has two lone pairs of nonbonding electrons. Moreover, it is surely bet-
ter to protonate on oxygen, and thus place the positive charge on the relatively elec-
tropositive carbon rather than to protonate on carbon, which would place the positive
charge on the highly electronegative oxygen atom.Finally, protonation at oxygen gives
a resonance-stabilized cation, whereas protonation at carbon does not, as shown in
Carbonyl hydration

776 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
Figure 16.24. So there is not really much choice in the direction of the protonation
step, and the result is a very electrophilic species, the protonated carbonyl compound.
In the second step of the reaction, water acts as a nucleophile and adds to the
Lewis acid (the protonated carbonyl) to generate a protonated diol (Fig. 16.25). In
the final step of this acid-catalyzed reaction, the protonated diol is deprotonated by
a water molecule to generate the neutral diol and regenerate the acid catalyst, H
3
O
.
A diol with both OH groups on the same carbon is called a gem-diol (gem stands
for geminal) or a hydrate. Note how closely related are the acid-catalyzed additions
of water to an alkene and to a carbonyl group. Each reaction is a sequence of three
steps: protonation, addition of water, and deprotonation.
H
H
protonation deprotonationaddition
The case for an alkene…
…and for a carbonyl
+
+
+
OH
2
..
+
H
2
O
..
..
OH
2
..
..
..
..
..
O
H
H
H
H
HO
Alcohol
Hydrate
..
H
3
O
H
protonation deprotonationaddition
+
+
OH
2
..
+
+
+
OH
2
..
..
..
..
..
..
H
2
O
..
..
O
OH
O
..
..
OH
H H
..
H
3
O
..
..
O
..
O
H
..
..
H
..
OH
(a)
(b)
FIGURE 16.25 A comparison of the
acid-catalyzed hydration reactions of
(a) an alkene and (b) a carbonyl
compound.
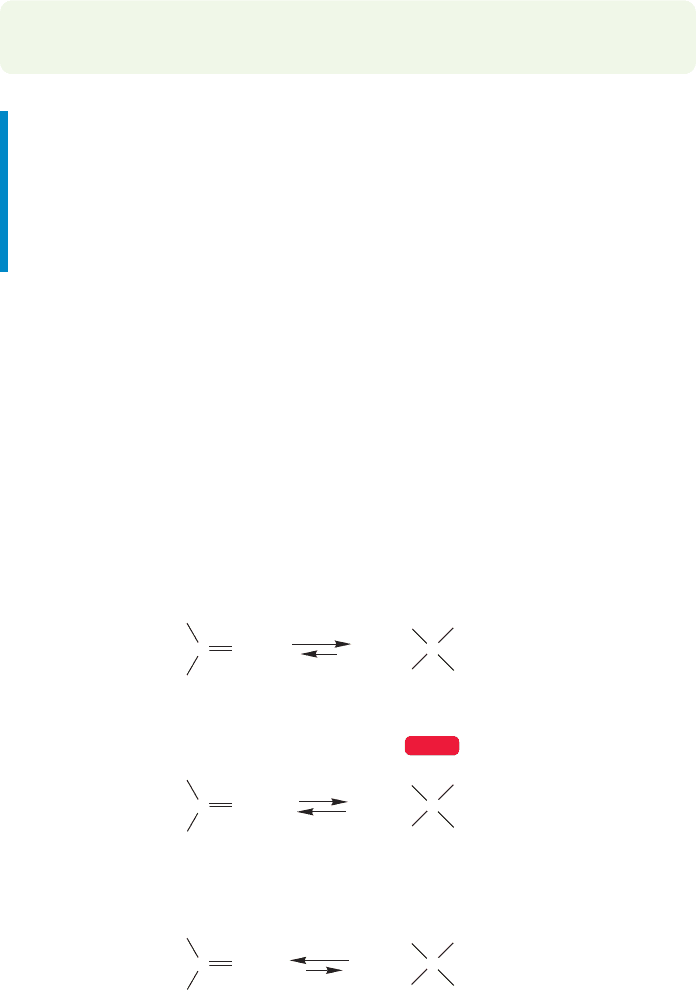
What about making the nucleophile more reactive? There is another mechanism
for hydration and it operates under basic conditions. Here the active agent is the
hydroxide ion,
OH. The first step in the base-catalyzed hydration reaction is
the addition of the strong nucleophile hydroxide to the carbonyl group (Fig. 16.26).
COH
..
..
The case for an alkene…
…and for a carbonyl
HO
..
..
HO
–
..
..
..
..
..
C
C
HO
–
–
–
..
C
C
HO
–
..
..
..
..
..
..
O
C
HO
O
C
..
..
H
O
H
Hydrate
+
..
..
..
OH
..
..
Hydroxide ion regenerated
FIGURE 16.26 Although strong bases
such as hydroxide ion do not add to
alkenes, they do attack carbonyl
groups.The final step in the base-
catalyzed hydration reaction is
protonation of the newly formed
alkoxide ion by water. Hydroxide is
regenerated.
16.7 Equilibrium in Addition Reactions 777
Unlike protonation, the first step in the acid-catalyzed reaction,this reaction has no
counterpart in the chemistry of simple alkenes. After addition,a proton is transferred
from water to the newly formed alkoxide, generating the hydrate and regenerating
the hydroxide ion.
Summary
The carbonyl group is hydrated under acidic (Fig. 16.25), basic (Fig. 16.26), and
neutral conditions (Figs. 16.20 and 16.23). In acid, the mechanism is accelerated
by protonation of the carbon–oxygen double bond to generate a strong electrophile
(Lewis acid).The relatively weak nucleophile,water, then adds.In base, the strong
nucleophile hydroxide adds to the relatively weak Lewis acid, the carbonyl group.
PROBLEM 16.8 Explain why bases such as the hydroxide ion do not add to the
oxygen of the carbon–oxygen double bond, nor to alkenes.
2.3 10
3
K
H
2
O
O
C
+
H
H
OHH
H
OH
C
1.06
H
2
O
O
C
Ethanal
(acetaldehyde)
Methanal
(formaldehyde)
+
H
OH
H
OH
C
2 10
–3
H
2
O
O
C
Acetone
+
OH
OH
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
WEB 3D
FIGURE 16.27 The equilibrium
constant for hydration of a
carbonyl-containing molecule can
favor either the hydrate or the
carbonyl compound.The
equilibrium constants shown
here are for pH 7 conditions.
Why should there be such great differences among these rather similar com-
pounds? First of all, remember that a little difference in energy goes a long way at
equilibrium (p.336). Recall also that alkyl groups stabilize double bonds (Table 3.1).
The more highly substituted an alkene, the more stable it is. The same phenom-
enon holds for carbon–oxygen double bonds. So, the stability of the carbonyl
16.7 Equilibrium in Addition Reactions
Carbonyl hydration reactions consist of several consecutive equilibria.In basic, acidic,
or even neutral water, simple carbonyl compounds are in equilibrium with their
hydrates,as Figure 16.27 shows.Even for molecules as similar as acetone and ethanal
(acetaldehyde), these equilibria settle out at widely different points. If we include
formaldehyde, the difference is even more striking.For these three molecules, which
differ only in the number of methyl groups attached to the carbonyl, there is a
difference in K of about a factor of 10
6
.
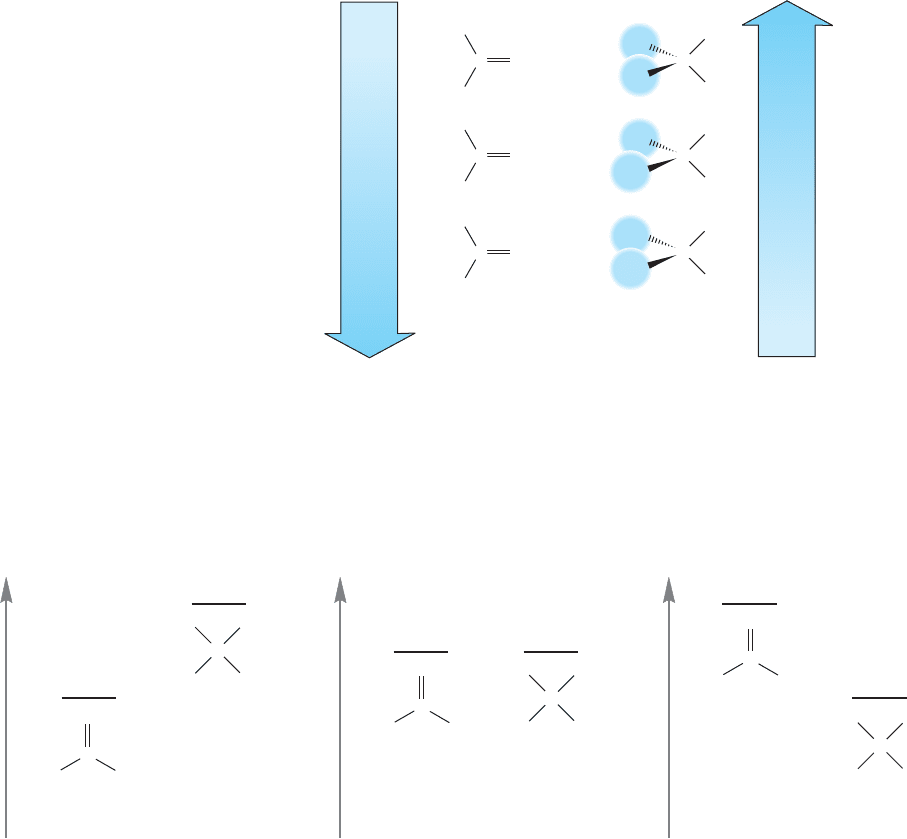
778 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
compound increases along the series: formaldehyde, acetaldehyde, acetone as the
substitution of the carbon–oxygen double bond increases (Fig. 16.28, left side).
If we now look at the molecule at the other side of the equilibrium, the hydrate,
we can see that increased methyl substitution introduces steric destabilization.
The more substituted the hydrate, the less stable it is (Fig. 16.28, right side). We
can immediately see the sources of the difference in K of 10
6
(Fig. 16.29). They
are the increased stabilization of the starting carbonyl compounds and destabi-
lization of the hydrates, both of which are induced by increased substitution by
methyl groups.
O
C
H
H
OH
OH
C
O
C
Increasing stability
Increasing stability
Note increased
substitution by
methyls
Note steric
destabilization
by methyls
O
C
H
3
C
H
3
C
H
H
OH
OH
C
H
H
3
C
OH
OH
C
H
3
C
H
H
3
C
H
3
C
FIGURE 16.28 Like alkenes, more
substituted carbonyl groups are more
stable than their less substituted
counterparts. In the hydrates,
increasing substitution results in
increasing destabilization through
steric interactions.
Energy
HO
H
3
C
OH
CH
3
+
C
Acetone is more stable and
its hydrate is less stable
K ~ 1K < 1 K > 1
The aldehyde and hydrate
are of comparable energy
Formaldehyde is less stable
and its hydrate is more stable
O
H
3
C
H
2
O
CH
3
Energy
HO
H
3
C
OH
H
+
C
O
H
3
C
H
2
O
H
Energy
C
HO
C
C
OH
HH
+
C
O
H
H
2
O
H
FIGURE 16.29 The magnitude of the equilibrium constant (K) depends on the relative energies of the carbonyl compounds
and their hydrates.We know that for acetaldehyde the energies of hydrate and aldehyde are comparable, because K 1.
Acetone is more stable than acetaldehyde and formaldehyde is less stable. Steric factors make the acetone hydrate less stable
than the acetaldehyde hydrate. By contrast, the formaldehyde hydrate is more stable than the acetaldehyde hydrate. The
equilibrium constants reflect these changes.
'
We can see other striking effects on this equilibrium process in Table 16.3. For
example, benzene rings, which can interact with the carbon–oxygen double bond by
resonance,decrease the amount of hydrate present at equilibrium. However,electron-
withdrawing groups on the α position adjacent to the strongly favor theC
P
O
