Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

16.7 Equilibrium in Addition Reactions 779
hydrate. There also appears to be a steric effect. Large alkyl groups favor the
carbonyl compound, as can be seen from the sequence of decreasing equilibrium
constants in Table 16.3: propanal butanal 2,2-dimethylpropanal. Each of these
phenomena can be understood by considering the relative stabilities of the starting
materials and products.
Energy
C
Model acetaldehyde (carbonyl and hydrate
are of comparable stability K ~ 1)
Aldehyde below hydrate
O
CH
3
O
Resonance stabilization of the aromatic aldehyde
[note use of (
+) to show resonance forms]
Hydrate (not stabilized
by resonance)
H
Energy
CH
3
H
H
–
O
..
..
..
..
..
..
..
H
2
O
+
(+)
(+)
C
Cl
O
H
C
Cl
H
C
Cl
H
C
Cl
C
HO
..
..
OH
..
..
H
Cl
HO
HO
..
..
..
..
..
OH
..
..
..
..
..
..
..
C
OH
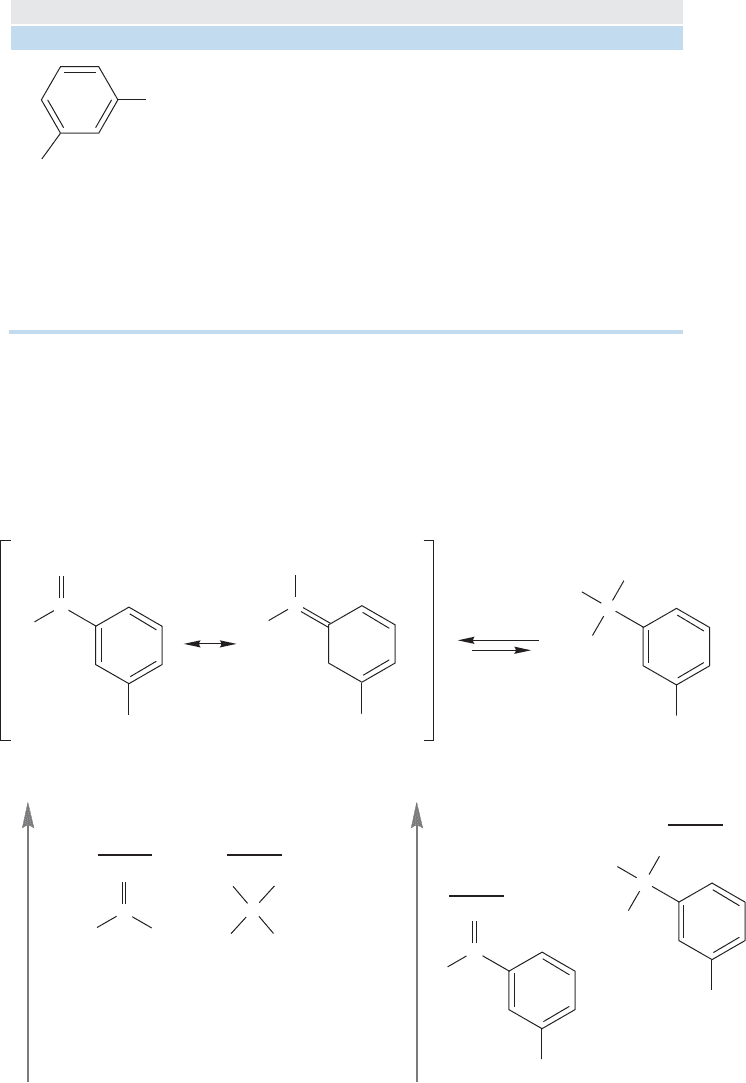
FIGURE 16.30 The aromatic
carbonyl compound is stabilized
by resonance, but the product
hydrate is not. Accordingly, the
carbonyl compound is favored at
equilibrium.
Resonance stabilization of the carbonyl group is generally not accompanied by a
resonance stabilization of the product hydrate (Fig. 16.30). For example, the aromatic
aldehyde, m-chlorobenzaldehyde (Table 16.3), is more strongly stabilized by resonance
than acetaldehyde. There is no corresponding difference in the hydrates, and there-
fore the aromatic aldehyde is less hydrated at equilibrium than acetaldehyde.
TABLE 16.3 Equilibrium Constants for Carbonyl Hydration
Structure Name K
CH
3
CH
2
CHO
CH
3
CH
2
CH
2
CHO
(CH
3
)
3
CCHO
CF
3
CHO
CCl
3
CHO
ClCH
2
CHO
m-Chlorobenzaldehyde
Propanal
Butanal
2,2-Dimethylpropanal
Trifluoroethanal
Trichloroethanal
Chloroethanal
0.022
0.85
0.62
0.23
2.9 × 10
4
2.8 × 10
4
37
Compound
CHO
Cl
780 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
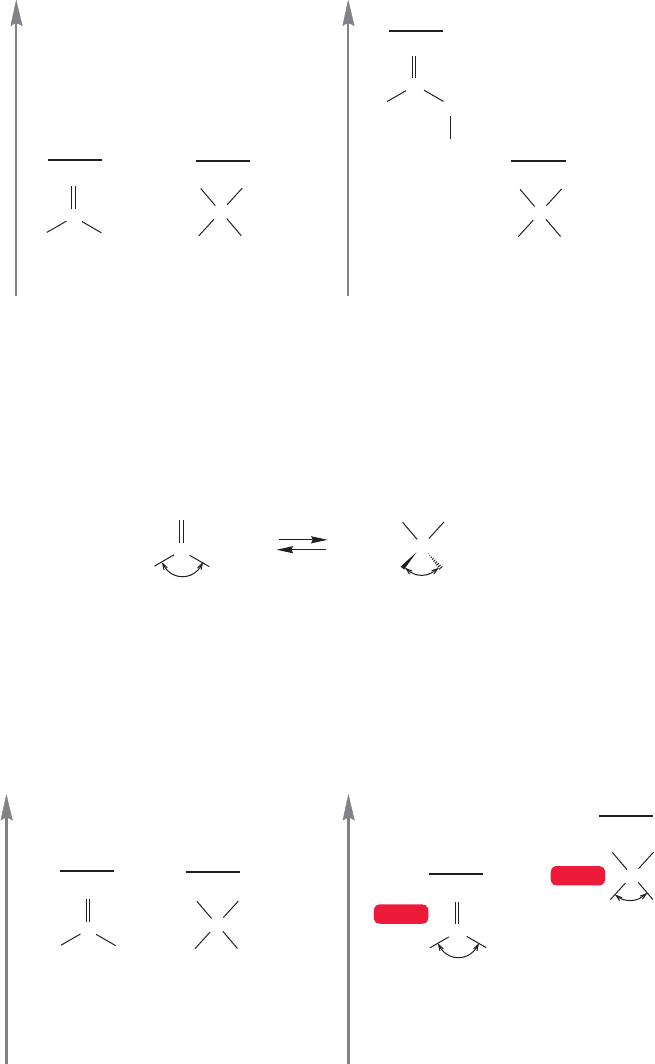
The reason electron-withdrawing groups such as chlorine or fluorine sharply
destabilize carbonyl groups when attached at the α position is that the dipoles oppose
each other. The carbon atom is already electron deficient, and further inductive
removal of electrons by the halogen on the adjacent carbon is costly in energy terms.
There is relatively little effect in the product hydrate, although there is some desta-
bilization in this molecule as well. So, as Figure 16.31 shows, the strong destabiliza-
tion of the carbonyl group favors the hydrate at equilibrium.
Energy
Energy
C
Model acetaldehyde (carbonyl and
hydrate are of comparable stability)
(K ~1)
The inductive effect of the electronegative
chlorine atom destabilizes the aldehyde
relative to the hydrate
O
CH
3 CH
3
H
H
O
H
C
C
HO
..
..
..
OH
..
CH
2
Cl
H
C
HO
..
..
..
OH
..
..
..
CH
2
Cl
..
..
..
..
..
..
δ
+
δ
+
δ
–
δ
–
FIGURE 16.31 Addition of a chlorine
in the α position of acetaldehyde
raises the energy of the carbonyl
compound.
The steric effect is slightly more subtle. In the carbonyl group, the carbon is
hybridized sp
2
and the bond angles are therefore close to 120° (Fig. 16.32). In
the product hydrate, the carbon is nearly sp
3
hybridized, and the bond angles
must be close to 109°. So, in forming the hydrate, groups are moved closer to each
C
O
R
H
R and H are
further apart
R and H are closer together
(destabilizing for large R)
H
R
C
HO OH
~120⬚ ~109⬚
..
..
..
..
....
FIGURE 16.32 In the hydrate, groups
are closer together than they are in
the carbonyl compound.This
proximity destabilizes the hydrate
relative to the carbonyl compound.
Energy
Energy
C
O
H
3
C
H
3
C
H
H
O
(CH
3
)
3
C
C
C
HO
..
..
..
OH
..
(CH
3
)
3
C
H
H
C
HO
..
..
OH
..
..
..
..
..
..
+
..
..
H
2
O
+
..
..
H
2
O
K ~1
K = 0.23
~120⬚
~109⬚
WEB 3D
WEB 3D
FIGURE 16.33 The larger the R
group, the more important the
steric effect. For example, 2, 2-
dimethylpropanal is less hydrated at
equilibrium than is acetaldehyde.
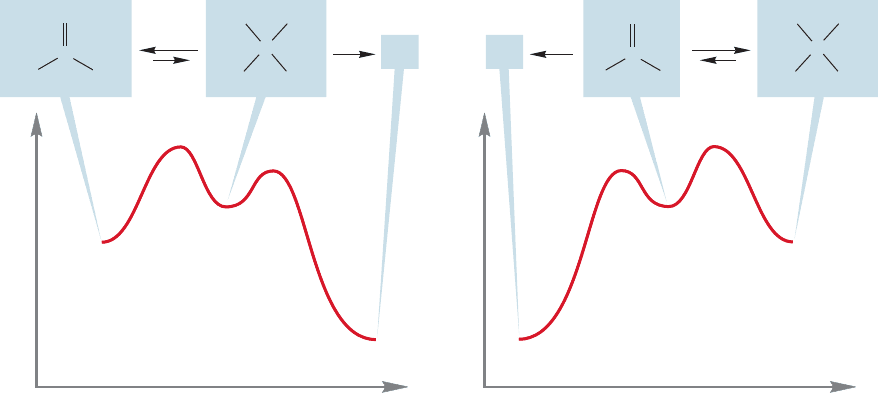
These equilibrium effects are important to keep in mind. Equally important is
the notion that as long as there is some of the higher energy partner present at equi-
librium, it can be the active ingredient in a further reaction.For example, even though
other, and for a large group that is relatively destabilizing. It is less costly in energy
terms to move a methyl group closer to a hydrogen than to move a tert-butyl group
closer to a hydrogen. Accordingly, acetaldehyde is more strongly hydrated than is
2,2-dimethylpropanal (pivaldehyde) (Fig. 16.33).
16.8 Other Addition Reactions: Additions of Cyanide and Bisulfite 781
Energy
Reaction progress
C
O
H
3
C
H
2
O
CH
3
H
3
C
C
X
HO OH
CH
3
Energy
Reaction progress
C
O
H
H
2
O
H
H
C
Y
HO OH
H
Here the unfavored hydrate
leads to product X
Here the unfavored carbonyl
compound leads to product Y
(a) (b)
FIGURE 16.34 Molecules not favored at equilibrium may nonetheless lead to products.These two examples show a
pair of carbonyl compound–hydrate equilibria in which it is the higher energy, unfavored partner that leads to product.
As the small amount of the higher energy molecule is used up, equilibrium is reestablished and more of the unfavored
compound is formed. (a) Reaction of the acetone hydrate is possible even though the hydrate is not the favored species.
(b) Reaction of the formaldehyde carbonyl group is possible even though the carbonyl is not the favored species.
As usual,mechanisms stick in the mind most securely if we are able to write them
backward.“Backward”is an arbitrary term anyway, because by definition an equilib-
rium runs both ways. Carbonyl addition reactions are about to grow more complex,
and you run the risk of being overwhelmed by a vast number of proton additions
and losses in the somewhat more complicated reactions that follow. Be sure you are
completely comfortable with the acid-catalyzed and base-catalyzed hydration reac-
tions in both directions before you go on. That advice is important.
it is a minor component of the equilibrium mixture (Fig.16.34a), the hydrate of ace-
tone could go on to other products (X), with more hydrate being formed as the small
amount present at equilibrium is used up. Similarly, even though it is extensively
hydrated in aqueous solution, reactions of formaldehyde to give Y can still be
detected (Fig.16.34b).It is also worth mentioning that gem-diols (hydrates) are very
difficult to isolate.Even though we know they exist in aqueous environment,in order
to isolate the diol we must remove the water and that drives the equilibrium toward
the carbonyl species.
16.8 Other Addition Reactions: Additions
of Cyanide and Bisulfite
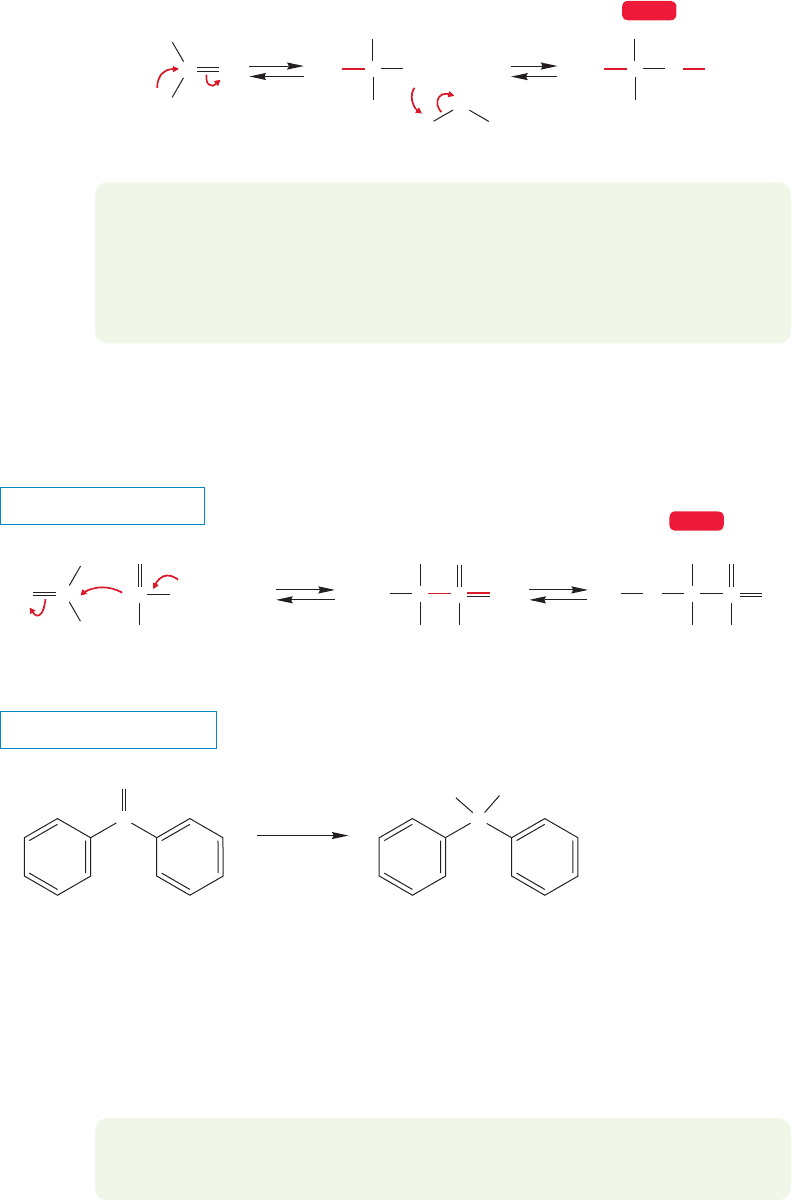
Many other addition reactions follow the pattern of carbonyl hydration. Two typical
examples are cyanohydrin formation and bisulfite addition. Both of these reactions are
generally base catalyzed.Because of an understandable and widespread aversion to work-
ing with the volatile and notorious HCN, cyanide additions are generally carried out
with cyanide ion.Potassium cyanide (K
CN) is certainly poisonous,but it’s not volatile,
and one knows where it is at all times,at least as long as basic conditions are maintained.
782 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
Cyanide ion is a good nucleophile and attacks the electrophilic carbonyl compound
to produce an alkoxide (Fig. 16.35). This alkoxide is protonated by solvent, often
water, to give the final product, a cyanohydrin. The cyanohydrin is often not stable
under basic conditions and reverses to the carbonyl compound and cyanide.
PROBLEM 16.9 Why does acetaldehyde in aqueous solution form an excellent
yield of cyanohydrin even though acetaldehyde is substantially hydrated in water
(K 1)? Might it not be argued that, because about one-half of the aldehyde is
present in hydrated form at equilibrium, no more than about 50% cyanohydrin
can be formed. Why is this argument wrong? Hint: See Figure 16.34.
'
–
C C
O
..
..
..
O
..
..
..
..
NC
..
NC
..
NC
–
C
O
..
..
O
..
..
A cyanohydrin
H
HH
–
OH
..
..
..
K
+
K
+
K
+
WEB 3D
FIGURE 16.35 Cyanohydrin formation.
Bisulfite addition to an aldehyde or ketone is similar to cyanide addition,although
the nucleophile in this case is sulfur. Sodium bisulfite adds to many aldehydes and
ketones to give an addition product, often a nicely isolable solid (Fig. 16.36).
OH OH
..
..
O
..
..
..
..
NaHSO
3
H
2
O
(98%)
HO
SO
3
Na
–
S
O
..
..
..
..
O
..
..
S
O
..
..
O
..
..
C
H
R
O
..
..
..
O
..
..
S
O
..
..
O
..
..
C
H
H
R
H
R
–
–
O
..
..
..
Na
+
Na
+
Na
+
C
C C
O
THE GENERAL CASE
A SPECIFIC EXAMPLE
WEB 3D
FIGURE 16.36 Bisulfite addition.
The reactions we have seen so far have all been simple additions of a generic HX
to the carbon–oxygen bond of a carbonyl group. Now we move on to related reac-
tions in which there is one more dimension. In these new reactions, addition of HX
is accompanied by the loss of some other group.
PROBLEM 16.10 Bisulfite addition is generally far less successful with ketones than
it is with aldehydes. Explain why.
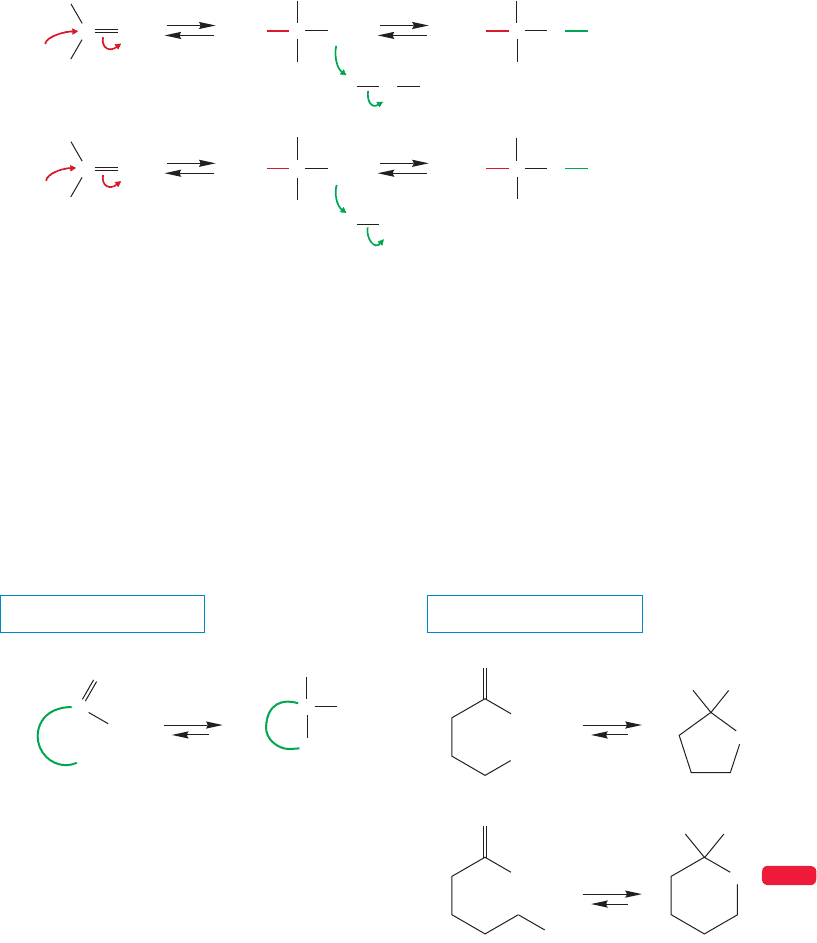
16.9 Addition Reactions Followed by Water Loss: Acetal Formation 783
16.9 Addition Reactions Followed by Water Loss:
Acetal Formation
If aldehydes and ketones form hydrates in water ( ), shouldn’t there be a
similar reaction in the presence of alcohols ( )? Indeed there should be,
and there is. However, the reactions are slightly more complicated than simple
hydration, at least under acidic conditions. In base, the two reactions are entirely
analogous, so let’s look at that reaction first.
Figure 16.37 contrasts the base-catalyzed reaction of a carbonyl compound in
water to give a hydrate with the related formation of a hemiacetal in an alcohol.
The hemiacetal is similar to a gem-diol, except that the central carbon has an OH
and an OR group rather than two OH groups.
R
O
OH
H
O
OH
–
C C
O
..
..
..
O
..
..
O
..
..
Hydrate
H
H
–
OH
..
..
..
–
HO
..
..
CH
O
..
..
HO
..
..
HO
..
..
..
+
–
C C
O
..
..
..
O
..
..
OR
..
..
Hemiacetal
H
–
OR
..
..
..
–
RO
..
..
CH
O
..
..
RO
..
..
RO
..
..
..
+
FIGURE 16.37 The reaction of a
carbonyl group with hydroxide ion
in water parallels the reaction of a
carbonyl with an alkoxide ion in
alcohol. In water, a gem-diol (or
hydrate) is formed; in alcohol, the
analogous product is called a
hemiacetal.
OH
..
..
OH
..
..
..
..
..
..
..
..
..
O
..
..
O
..
(88.6%)(11.4%)
(93.9%)(6.1%)
C
H
H
Open-chain
hydroxy–aldehyde
Cyclic
hemiacetal
C
OH
H
O
OH
H
O
O
O
H
HO
H
HO
THE GENERAL CASE
SPECIFIC EXAMPLES
WEB 3D
H
2
O H
2
O
H
2
O
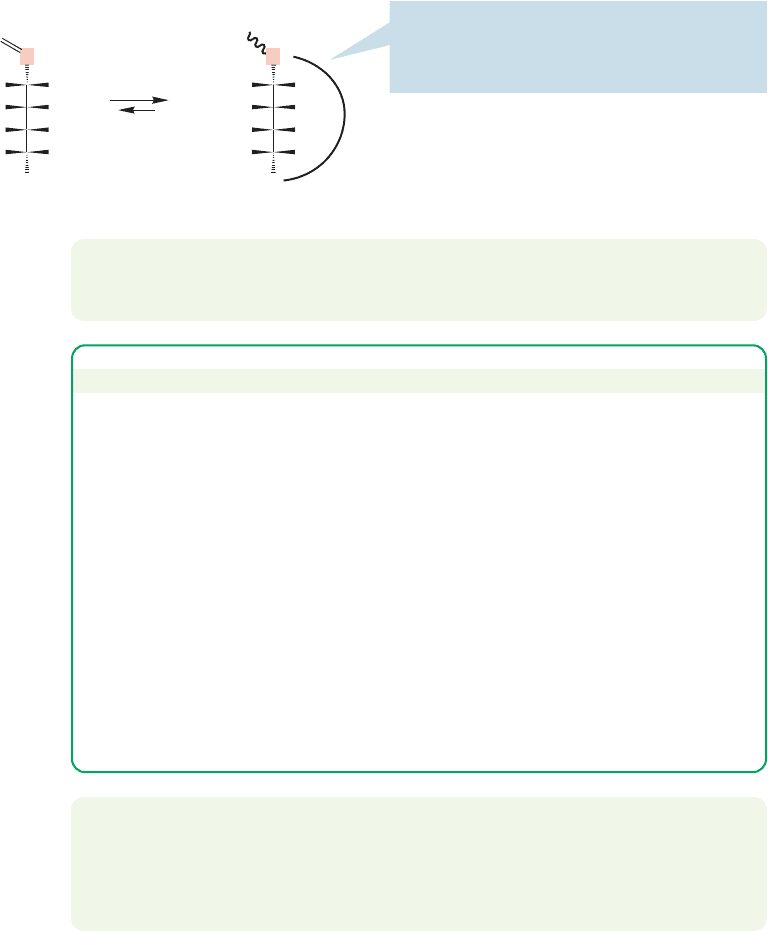
FIGURE 16.38 Cyclic hemiacetals are often more stable than their open-chain counterparts.
Hydration and hemiacetal formation are comparable, so it is no surprise that
the discussion in Section 16.7 on equilibrium effects is quite directly transferable to
this reaction. Aldehydes, for example, are largely in their hemiacetal forms in basic
alcoholic solutions (in acid the reaction goes further, as we will see), whereas ketones
are not.
Most simple hemiacetals, like gem-diols, are not isolable. However, the cyclic
versions of these compounds often are. Consider combining the carbonyl and
hydroxyl components of the hemiacetal reaction in Figure 16.37 within the same
molecule (Fig. 16.38). Now hemiacetal formation is an intramolecular process. If
the ring size is neither too large nor too small, the hemiacetals are often more sta-
ble than the open-chain molecules.
784 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
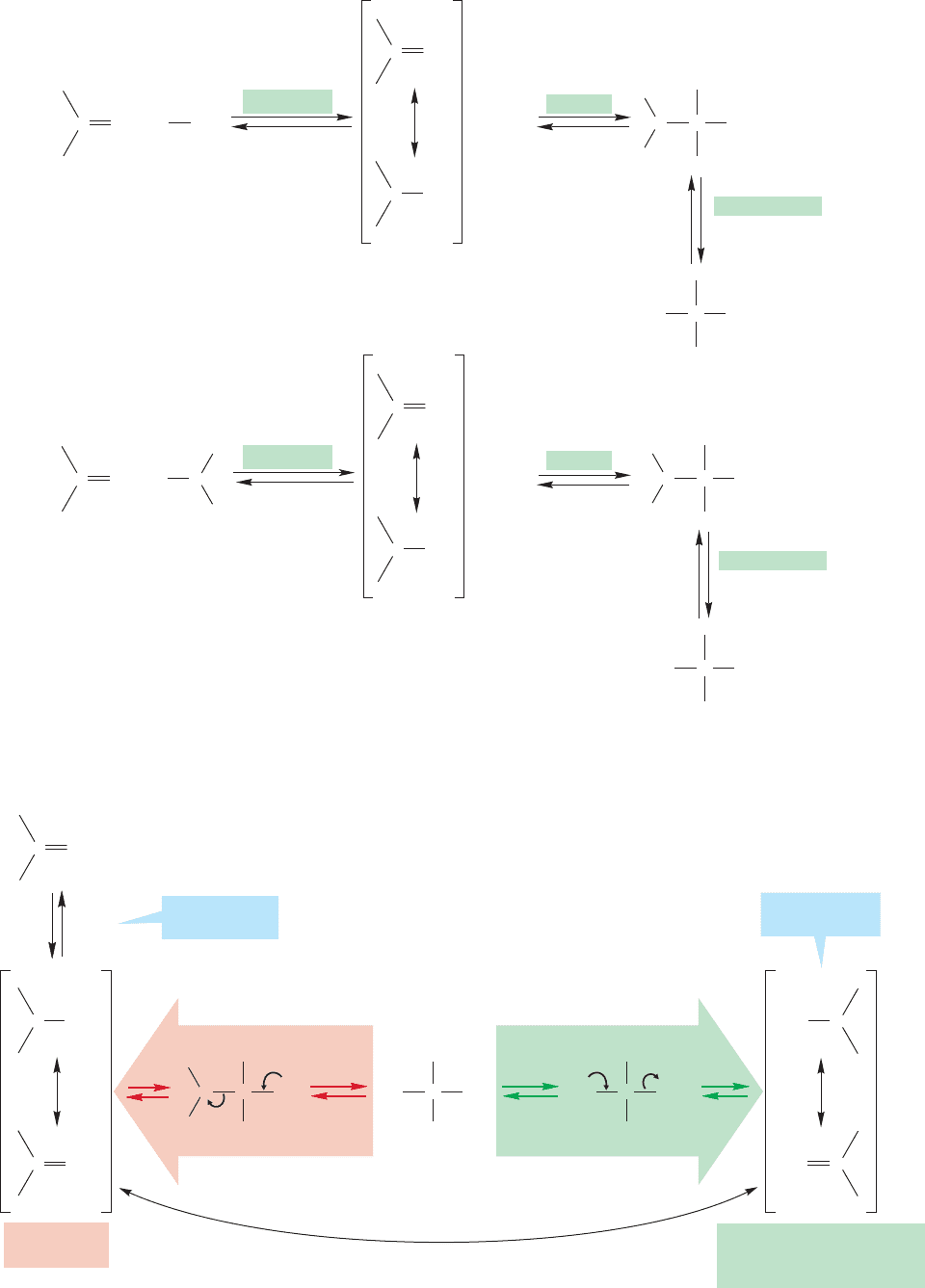
Now, what about the acid-catalyzed version of the reaction of alcohols and alde-
hydes or ketones? The process begins with hemiacetal formation, and the mecha-
nism parallels that of acid-catalyzed hydration (Fig. 16.25b). A three-step sequence
of protonation, addition, and deprotonation occurs in each case (Fig. 16.40).
Can anything happen to a hemiacetal in acidic solution? Does the reaction stop there?
One thing you know happens is the set of reactions indicated by the reverse arrows in
the equilibria of Figure 16.40.In this case, the oxygen atom of the OR is protonated and
an alcohol molecule is lost to give the resonance-stabilized protonated carbonyl com-
pound.This intermediate is deprotonated to give the starting ketone or aldehyde.There
is no need for us to write out the mechanism because it already appears exactly in the
reverse steps of Figure 16.40. It is simply the forward reaction run directly backward.
PROBLEM SOLVING
Problems that ask you to write a mechanism for the transformation of a starting
material into a product are common,and they will appear increasingly throughout the
remaining chapters.This particular problem should be relatively easy, but it serves as
an example of how to analyze what must happen.That kind of analysis is absolutely
critical to success in solving hard mechanistic problems. It is very, very important to
get into the habit right now of defining goals in mechanism problems. For example,
in the first part of this problem, what happens? A carbon–oxygen bond is made. A
carbonyl group becomes an alcohol. So, we have two goals (write them down!):
1. Close the ring by making a bond.
2. Break the double bond.
It is easy to see that the two goals are connected—this is an easy problem—but
defining and paying attention to those goals is critical practice, and a great habit
to get into.
C
P
O
C
O
O
HOCH
2
The sugar, D-glucose
H
H
H
H
OH
OH
OH O
Open form
HO
O
CH
HOCH
2
H
H
H
H
OH
OH
Cyclic hemiacetal form
HO
HO
CH
Watch out, there is a convention at work here;
there is no “corner” in the oxygen bridge; sugar
chemists have learned to reinterpret the long
curved bond in the bottom part of the structure
FIGURE 16.39 Sugars exist mainly
in their cyclic hemiacetal forms.
The most spectacular examples of this effect are the sugars, polyhydroxylated
compounds that exist largely, though not quite exclusively, in their cyclic hemiacetal
forms (Fig. 16.39). We will explore the chemistry of sugars in Chapter 22.
PROBLEM 16.11 Write a mechanism for the intramolecular formation of one of the
hemiacetals of Figure 16.38.
PROBLEM 16.12 Note how odd the cyclic structure in Figure 16.39 looks.
Construct a three-dimensional picture of
D-glucose that better represents its
structure in the cyclic hemiacetal form. Note that the position of the OH on the
carbon marked in red in Figure 16.39 can be either axial or equatorial.
16.9 Addition Reactions Followed by Water Loss: Acetal Formation 785
H
2
O
..
..
O
C
O
..
..
..
..
C
..
H
OH
2
H
H
COH
..
..
HO
..
..
Hydrate
COH
..
..
COH
..
..
..
HO
..
..
+
+
+
H
2
O
+
+
..
OH
+
C
..
..
OH
+
Hydration reaction in acid
addition
protonation
deprotonation
H
3
O
..
+
FIGURE 16.40 The acid-catalyzed
formation of a hemiacetal parallels
the acid-catalyzed hydration reaction.
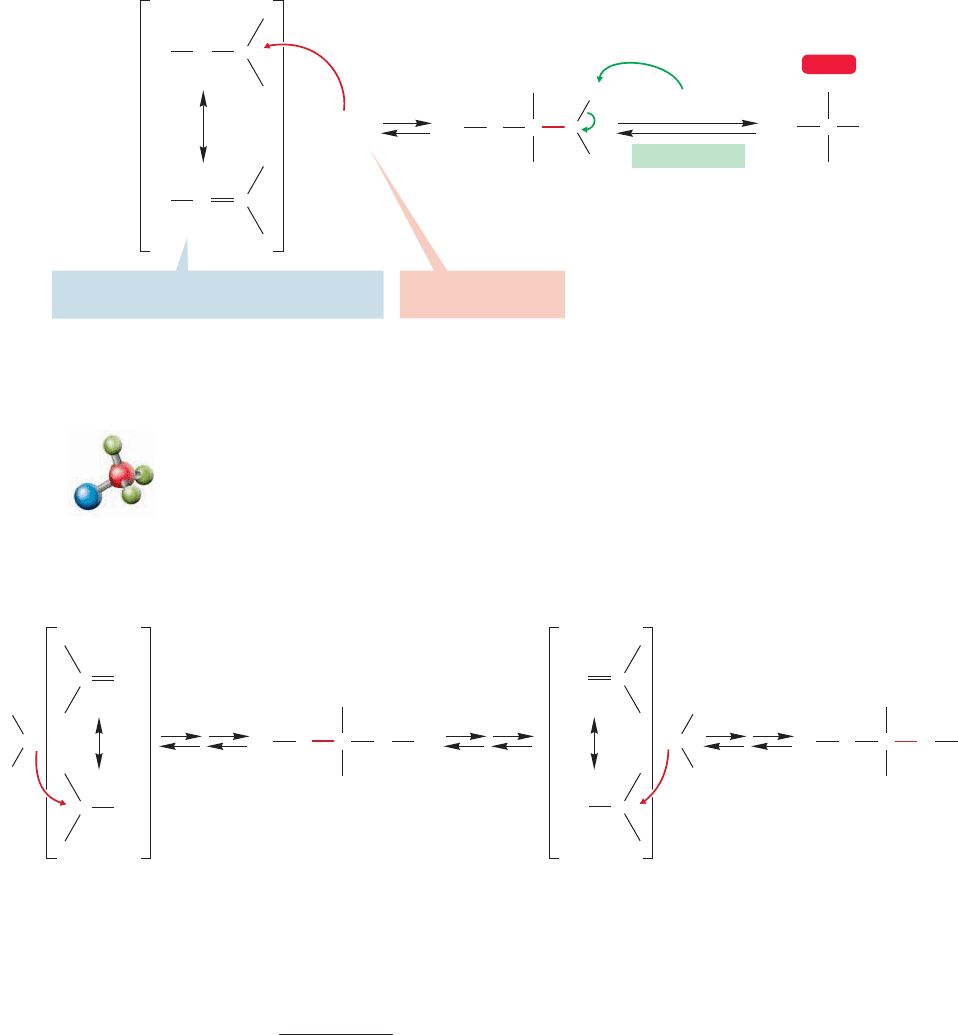
But suppose it is not the OR oxygen that is protonated, but
instead the OH oxygen? Surely, if one of these reactions is
possible, so is the other. If we keep strictly parallel to the
reverse process, we can now lose water to give a resonance-
stabilized species (Fig. 16.41), which is quite analogous to the
COH
..
..
RO
..
..
Hemiacetal
COH
..
..
....
..
COH
2
..
RO
..
..
C
..
..
..
RO
..
..
..
..
+
+
C
..
OH
+
C
..
..
OH
+
C
..
..
O
A protonated
carbonyl
This resonance-stabilized
intermediate is analogous
to the protonated carbonyl
ROH
..
..
ROH
2
..
+
ROH
2
..
+
O
R
H
COH
+
C
+
RO
....
C
+
+
ROH
2
..
+
No proton on
oxygen to lose!
Deprotonation
step
Protonation of the OR
Protonation of the OH
..
..
H
2
O
FIGURE 16.41 Protonation of the OH in
the hemiacetal, followed by water loss,
leads to a resonance-stabilized species that
is analogous to the protonated carbonyl.
..
..
..
..
..
..
Hemiacetal formation in acid
addition
protonation
HOR
..
..
HOR
..
..
..
O
C
H
R
O
..
..
O
..
C
H
H
R
COH
RO
Hemiacetal
COH
COH
+
+
H
2
OR
..
+
deprotonation
+
+
+
..
OH
+
C
..
..
OH
+
786 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
Notice that the second addition of alcohol in acetal formation is just like the first;
the only difference is the presence of an H or R group in the species to be attacked
(Fig. 16.43). Notice also that acetal formation is completely reversible.
..
..
..
..
C
+
RO
RO
....
C
+
CO
..
..
O R R
..
..
C
..
..
R
H
First alcohol addition Second alcohol addition
CO
..
..
O
..
..
CHR
..
..
C
..
OH
+
C
..
..
OH
+
O
R
H
Resonance-stabilized
intermediate
(a protonated carbonyl)
Hemiacetal Acetal
Resonance-stabilized
intermediate (R
replaces H)
..
..
O
..
..
FIGURE 16.43 The two alcohol additions that occur in acetal formation are very similar to each other.
resonance-stabilized protonated carbonyl compound of Figure 16.40. But now depro-
tonation is not possible—there is no proton on oxygen to lose. Another reaction occurs
instead,the addition of a second molecule of alcohol.Deprotonation gives a full acetal
2
(Fig.16.42).An acetal contains an sp
3
hybridized carbon that has two OR groups attached.
An acetal
ROH
..
..
O
..
..
..
..
+
C
+
O
R
R
R
....
C
+
This species cannot lose a proton to
regenerate the carbonyl compound, so…
…addition of a
second HOR occurs
HOR
..
..
+
..
..
..
O
H
R
C
+
COR
..
..
RO
..
..
C
..
..
O
deprotonation
ROH
2
..
+
WEB 3D
FIGURE 16.42 An acetal is formed by addition of a second molecule of alcohol to the resonance-stabilized intermediate.
2
Organic chemists originally used hemiketal and ketal for describing the products from alcohol addition to
ketones; and hemiacetal and acetal were used for the products from alcohol addition to aldehydes.The IUPAC
system has dropped the hemiketal and ketal names in favor of using hemiacetal and acetal for the species formed
from both aldehydes and ketones. We will not use hemiketal or ketal, but be alert, because one still sees these
sensible terms occasionally.
Acetal formation
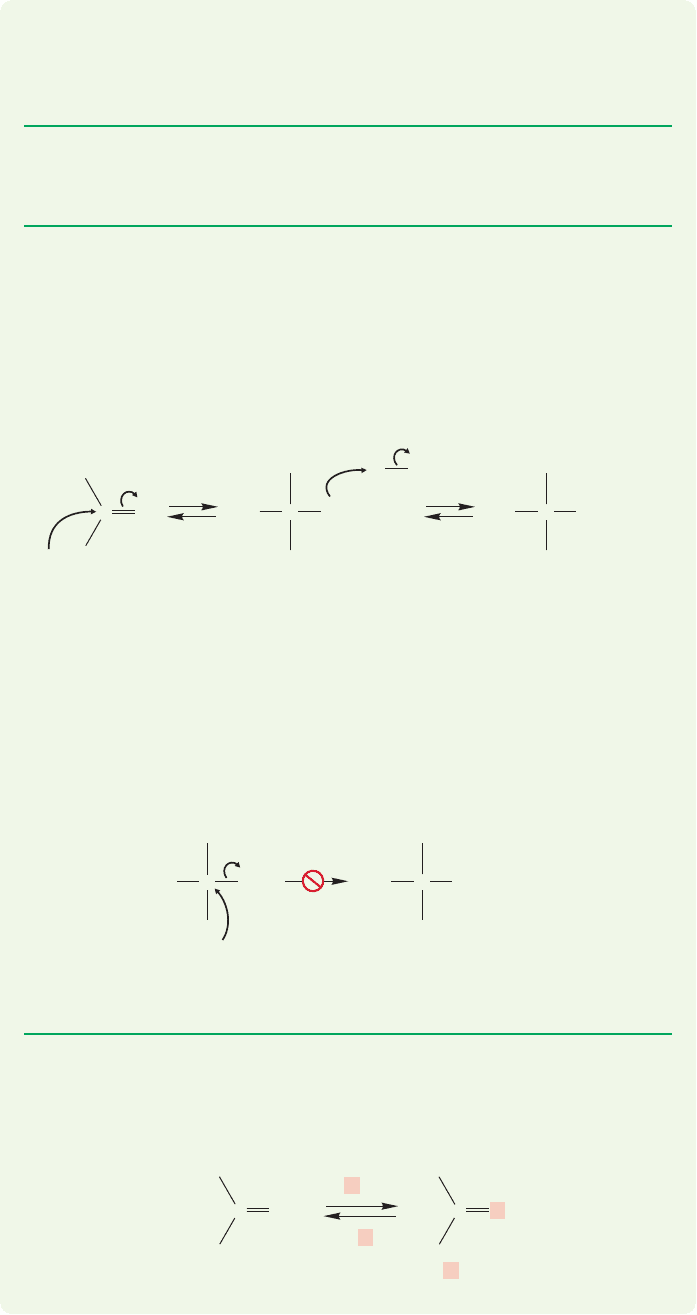
16.9 Addition Reactions Followed by Water Loss: Acetal Formation 787
PROBLEM 16.13 Write the mechanism for the formation of the acetal from
treatment of cyclopentanone in ethylene glycol (1,2-ethanediol) with an acid
catalyst.
PROBLEM 16.14 Write the mechanism for the reverse reaction, the regeneration of
the starting ketone on treatment of the acetal with aqueous acid.
WORKED PROBLEM 16.15 Explain why hemiacetals, but not full acetals, are formed
under basic conditions.
ANSWER If we work through the mechanism we can see why. The first step is
addition of an alkoxide to the carbonyl group to form the alkoxide intermediate.
Protonation then gives the hemiacetal and regenerates the base.
..
..
O
..
..
..
RO
C
..
..
..
O
..
..
RO
..
..
OR
H
HemiacetalAlkoxide
C
..
..
OH +
..
..
RO
C
–
–
..
..
..
OR
–
..
..
RO
C
..
..
OR
..
..
OH +
..
..
RO
C
..
..
..
OH
..
..
RO
–
–
PROBLEM 16.16 When acetone is treated with
18
OH
2
/
18
OH
3
, acetone is found to
be labeled with
18
O. Explain.
O
C
..
..
O
C
..
..
O =
18
O
..
..
OH
2
H
3
O
..
+
Now what? There is no acid available to protonate the OH oxygen. In order to
get an acetal, RO
must displace hydroxide! This reaction is most unfavorable.
The more favorable reaction between RO
and the hemiacetal is removal of the
proton to re-form the intermediate alkoxide. In base, the reaction cannot go
beyond hemiacetal formation.
788 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
excess ROH
acid catalyst
acid catalyst
excess H
2
O
CH
3
OH
2
CH
3
OH
+
+
O
C
X
C
X
OR
OR
C
Y
OR
OR
base
O
Desired conversion
C
Y
O
C
Y
chemistry
An acetal (stable in base);
the carbonyl group is
protected, or masked
Here the C
O
is unmasked
(regenerated)
O
A SPECIFIC EXAMPLE
THE GENERAL CASE
O
CH
3
O OCH
3
H
2
/Pd H
3
O
H
2
O
CH
3
O OCH
3
FIGURE 16.44 A protecting group
can be used to mask a sensitive
functional group while reactions are
carried out on other parts of the
molecule. When these reactions are
completed, the original functionality
can be regenerated.
Summary
By now, you may be getting the impression that almost any nucleophile will add to
carbonyl compounds. If so, you are quite right. So far, we have seen water, cyanide,
bisulfite, and alcohols act as nucleophiles toward the Lewis acidic carbon of the car-
bon–oxygen double bond.Addition can take place under neutral,acidic,or basic con-
ditions. All of the addition reactions are similar, with only the details being different.
Those details are important though, as well as highly effective at camouflaging the
essential similarity of the reactions.We’ll now see some examples of acetal chemistry.
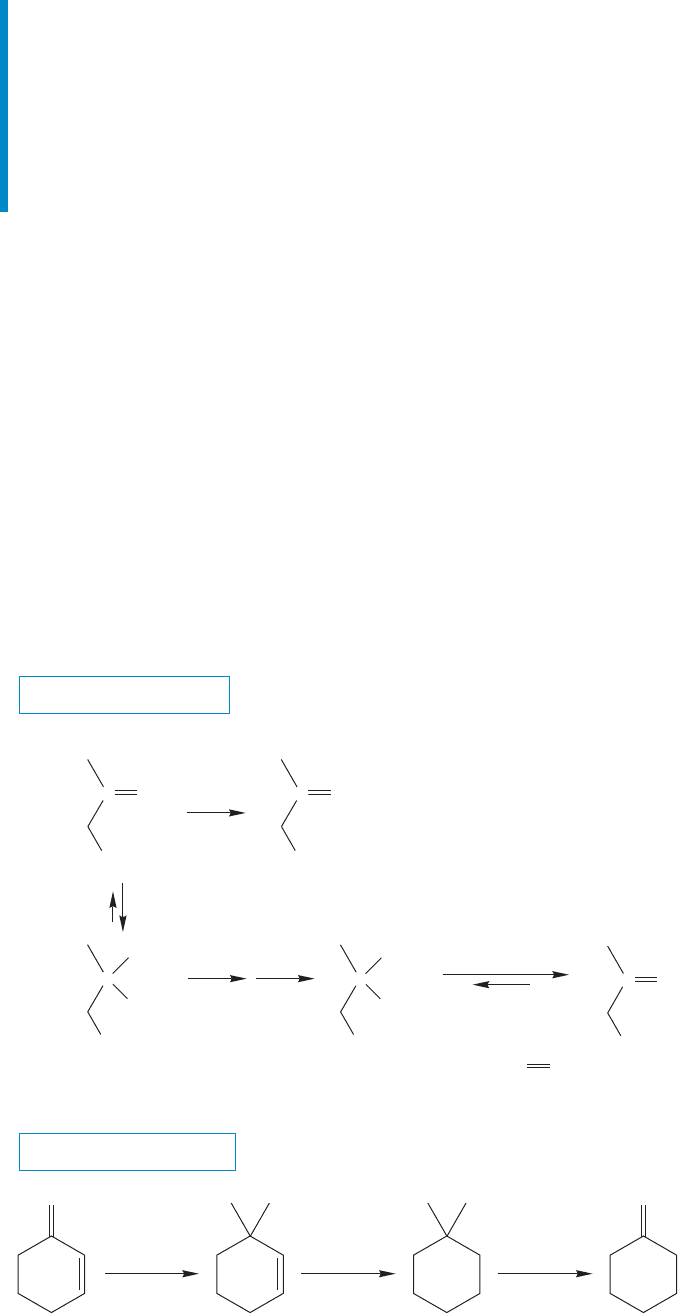
16.10 Protecting Groups in Synthesis
16.10a Acetals as Protecting Groups for Aldehydes and Ketones
By all means work out the mechanism of acetal formation by setting yourself the
problem of writing the mechanism for the reverse reaction (go back and do
Problem 16.14!), the formation of a ketone or aldehyde from the reaction of an
acetal in acidic water. Acetal formation is an equilibrium process, and conditions
can drive the overall equilibrium in the forward or backward direction. In an
excess of alcohol, an acid catalyst will initiate the formation of the acetal, but in
an excess of water, that same acid catalyst will set the reverse reaction in motion,
and we will end up with the carbonyl compound. This point is important and
practical. Carbonyl compounds can be “masked,” stored or protected, by convert-
ing them into their acetal forms, and then regenerating them as needed. The
acetal constitutes your first protecting group. For example, it might be desirable
to convert group X, in the carbonyl-containing molecule shown in Figure 16.44,
