Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

16.10 Protecting Groups in Synthesis 789
into group Y through a base-catalyzed reaction. But we know that carbonyl com-
pounds are sensitive to base, and the starting material may be destroyed in the
attempted reaction. The solution is first to convert the carbonyl group into an
acetal (which is unreactive in base), then carry out the conversion of X to Y, and
finally regenerate the carbonyl group.
16.10b Protecting Groups for Alcohols It is often desirable to protect a
hydroxyl group so that further reactions in base can take place without complica-
tion. One widely used method is to convert the hydroxyl group into a tetrahy-
dropyranyl (THP) ether through reaction with dihydropyran (Fig. 16.45). The
mechanism involves protonation of dihydropyran to give the resonance-stabilized
cation, which is then captured by the alcohol. The result is an acetal, a molecule
stable to base, but not to acid (p. 783). Regeneration of the alcohol can be accom-
plished through acid-catalyzed hydrolysis.
+
O
H
H
R
H
..
..
..
..
H
3
O / H
2
O
O
Dihydropyran
+
+
+
+
+
+
..
..
acid catalyst
15.5 h, 25 ⬚C
O
..
..
..
..
H
O
..
..
..
..
O
H
O
..
..
..
..
R
H
..
H
ROH
ROH
ROH
Alcohol to be
protected
A THP ether—an acetal
and a protected alcohol
The
regenerated
alcohol
..
..
+ ROH
2
..
O
..
RO
H
O
O
(97%)
..
..
..
..
HO
OH
O
O
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 16.45 The use of THP ethers as protecting groups for alcohols.
Another type of protecting group for alcohols is a silyl ether. A silyl ether
has the general formula of . The reaction of an alcohol with
a trialkylsilyl halide leads to a trialkylsilyl ether in near-quantitative yield
R¿
O
O
O
SiR
3
790 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
16.11 Addition Reactions of Nitrogen Bases: Imine
and Enamine Formation
The nitrogen atom of an amine (RNH
2
) is a nucleophile, and like the related oxy-
gen nucleophiles, it can add to carbonyl compounds. Indeed, the acid-catalyzed reac-
tion between a carbonyl compound and an amine to give a carbinolamine is quite
analogous to the reactions of water and alcohols we have just seen (Fig. 16.47).
CH
2
OH
+
NH
4
F
–
CH
2
O
(89%)
(100%)
(100%)
HCl
+
+
25 ⬚C
CH
2
Cl
2
CH
3
CH
3
(CH
3
)
3
C
CH
2
OH
Si Cl
CH
3
CH
3
Si C(CH
3
)
3
F
CH
3
CH
3
Si C(CH
3
)
3
FIGURE 16.46 The use of trialkylsilyl
ethers as protecting groups for
alcohols.
COH
..
..
RO
..
..
COH
..
..
..
..
+
C
..
..
OH
+
C
..
..
OH
+
Protonation
steps
Deprotonation
steps
Addition
steps
Hemiacetal
ROH
..
..
ROH
..
..
....
..
O
R
H
COH
+
R
NH
3
+
C
+
ROH
2
..
+
C
+
ROH
2
..
+
COH
..
..
COH
..
..
..
+
Carbinolamine
..
..
N
R
H
H
H
COH
+
RNH
3
+
RNH
2
..
RNH
2
R
N
..
O
..
O
..
..
HOH
..
..
C
+
HOH
2
..
+
COH
..
..
HO
..
..
COH
..
..
HO
..
..
+
Hydrate
(gem-diol)
HOH
..
..
....
..
O
H
H
COH
+
+
..
H
OH
2
..
..
O
FIGURE 16.47 Analogous reactions of carbonyl compounds give hydrates (called gem-diols), hemiacetals,
and carbinolamines. The usual sequence of protonation, addition, and deprotonation steps is followed.
(Fig. 16.46). The reaction depends for its success on the enormous strength of the
silicon–oxygen bond ( 110 kcal/mol).The silyl group can be removed through reac-
tion with fluoride ion or hydrogen fluoride. The silicon–fluorine bond is even
stronger ( 140 kcal/mol) than the silicon–oxygen bond, and the formation of the
trialkylsilyl fluoride provides the thermodynamic driving force for the reaction.
So, alcohols can be protected by converting them into THP or silyl ethers, and
regenerating them when needed.
'
'
16.11 Addition Reactions of Nitrogen Bases: Imine and Enamine Formation 791
Although the amine is more basic than the carbonyl oxygen, there still must be
sufficient protonation of carbonyl and availability of the free amine under the acid-
catalyzed conditions.
PROBLEM 16.17 Write the base-catalyzed versions of the reactions in Figure 16.47.
Like the hemiacetal, the carbinolamine is not a final product in acid. Further
steps in the two reactions are all similar. An OH is protonated, and then lost as
water (note that
OH is not the leaving group) to give a resonance-stabilized cation
(Fig. 16.48).
COH
..
..
RO
..
..
OH
..
..
..
..
Resonance-stabilized
cations
Hemiacetal
ROH
2
..
+
OH
..
..
OH
..
..
..
+
Carbinolamine
H
RNH
3
+
OH
..
..
HO
..
..
OH
..
..
HO
..
..
Hydrate
..
..
..
RO
+
H
H
N
R
H
+
..
HOH
2
R
O
..
..
R
O
+
C
..
R
O
+
C
..
..
..
O
H
+
H
H
O
..
+
H
H
O
+
..
C
C
+
..
..
H
O
+
C
..
H
O
+
C
N
R
H
N
R
H
+
+
N
..
..
H
2
O
..
..
H
2
O
..
..
H
2
O
C
CC
CC CC
CC
CC
..
FIGURE 16.48 Continuation of the reactions from Figure 16.47. Loss of water leads
to a resonance-stabilized intermediate in all three reactions.
792 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
Imine
Carbonyl
ROH
..
..
..
..
RNH
2
..
..
..
RO
C
+
H
R
..
..
HOH
O
..
..
R
O
+
C
..
R
O
+
C
..
C
C
..
..
+
C
..
..
O
+
C
..
O
+
C
..
..
O
C
N
R
N
R
+
C
N
R
+
+
+
RNH
3
+
COR
..
..
RO
..
..
C
..
..
..
..
Acetal
H
3
O
..
+
Here there is no proton that
can be lost from oxygen
In these cases, there is a proton
that can be lost from N or O
H
H
H
H
WEB 3D
FIGURE 16.49 Continuation of
reactions from Figure 16.48. In the
reaction with water, loss of a proton
leads to the recovered carbonyl
compound. In the alcohol reaction,
there is no proton to be lost, and
a second molecule of alcohol adds
to give an acetal. In the reaction
with amine, loss of a proton leads
to an imine.
In the acid-catalyzed reaction with alcohol shown in Figures 16.47 and 16.48,
the resonance-stabilized cation reacts by adding a nucleophile, ROH, because R
cannot usually be lost. Instead, the cation goes on to acetal formation by a second
addition of alcohol. For the water and amine additions, however, there remains an
available hydrogen on the resonance-stabilized cation that can be lost as a proton,
as shown in Figure 16.49. In the hydration reaction, this loss simply completes the
re-formation of the carbonyl group of the starting material, but in the amine exam-
ple it leads to the formation of a carbon–nitrogen double bond, called either a Schiff
base or an imine (Fig. 16.49).
There is a vast variety of imines formed from primary amines (RNH
2
,
monosubstituted derivatives of ammonia, NH
3
), and each has its own common
name. Many of them were once useful in analytical chemistry because they are
generally solids, and therefore could be characterized by their melting points.
Imine formation
16.11 Addition Reactions of Nitrogen Bases: Imine and Enamine Formation 793
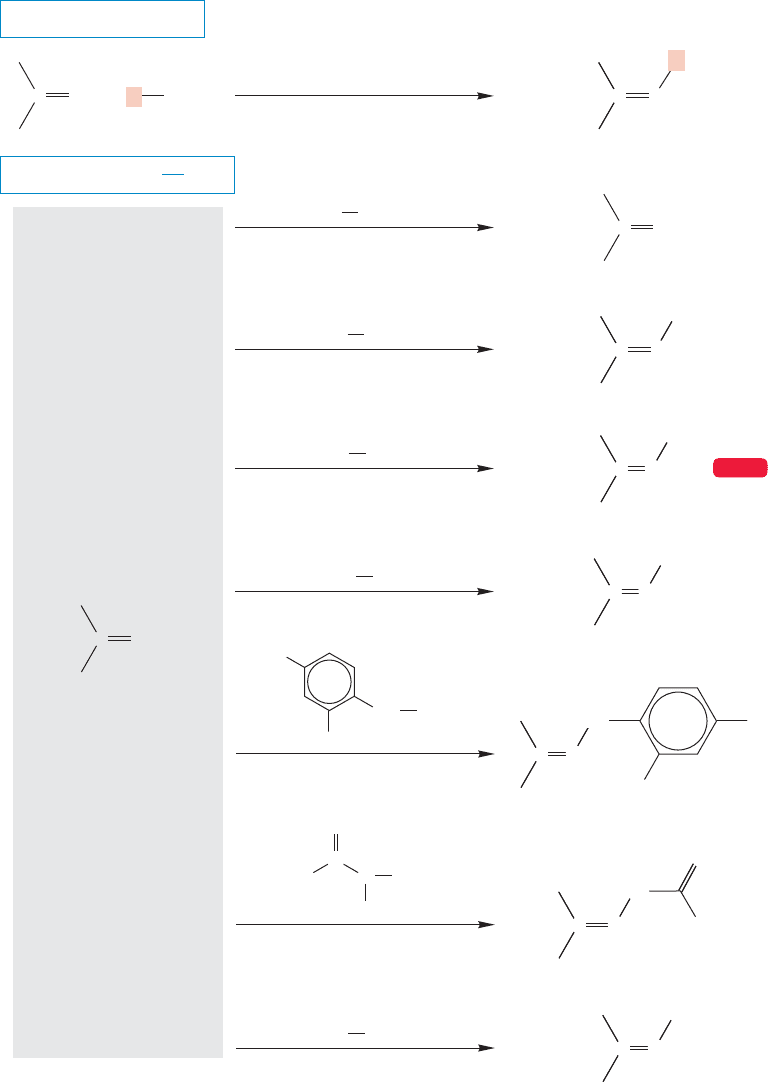
For example, formation of a 2,4-dinitrophenylhydrazone or a semicarbazone
(Fig. 16.50) from an unknown was not only diagnostic for the presence of a car-
bonyl group, but it yielded a specific derivative that could be compared to a similar
sample made from a compound of known structure. Figure 16.50 gives a few of
these substituted imines. Be sure to note that all of these reactions are the same—
the only difference is the identity of the R group.If you know one,you know them
all—but you have to know that one.
..
NH
2
+
..
..
O
C
..
C
N
R
..
..
O
C
C
Ph
..
C
..
C
N
..
NH
2
acid catalyst
H
..
NH
2
Ph
..
C
NH
Imine
Phenyl imine
C
N
..
C
N
.. ..
NH
2
H
2
N
Hydrazone
..
..
C
N
..
C
N
NHPh
.. ..
NH
2
PhHN
Phenylhydrazone
..
C
N
..
C
N
NH
.. ..
NH
2
2,4-Dinitrophenylhydrazone
..
..
C
..
C
N
NH
....
..
NH
2
..
NH
2
H
2
NN
H
C
O
Semicarbazone
..
..
C
N
..
C
N
.. ..
..
NH
2
HO
Oxime
..
NO
2
O
2
N
HN
NO
2
O
2
N
..
..
..
O
OH
NH
2
R
THE GENERAL CASE
EXAMPLES OF R NH
2
WEB 3D
FIGURE 16.50 Formation of some substituted imines.
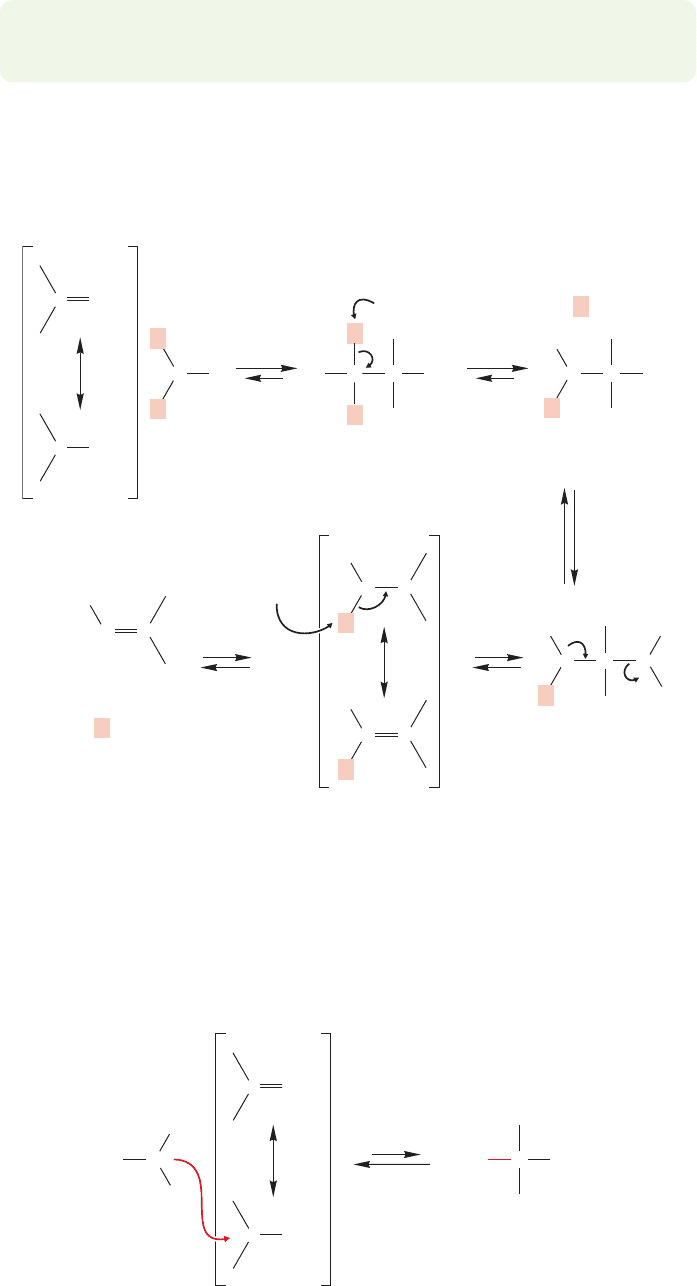
What happens if one or both of these hydrogens is missing? Tertiary amines
(R
3
N) carry no hydrogens and undergo no visible reaction with carbonyl groups.But
this masks reality. In fact, addition to the carbon–oxygen double bond does take place,
but the unstable intermediate has no available reaction except reversal to starting
material (Fig. 16.52).
794 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
First loss of
a hydrogen
Second loss of
a hydrogen
..
N
H
H
R
+
HNH
2
R
+
HNH
2
R
+
COH
..
..
..
COH
..
..
Carbinolamine
protonation
H
COH
..
..
COH
..
..
H
H
RN
..
..
..
C
C
N
R
H
N
R
H
+
+
C
..
OH
+
C
..
..
OH
+
R
N
R
H
C
.. ..
+
H
H
O
RNH
2
C
N
R
An imine
N
NH
2
R
..
FIGURE 16.51 In order to form an
imine, there must be two hydrogens
(red) on the starting amine.That is,
the amine must be either primary
(RNH
2
) or ammonia (NH
3
).
No H on N to lose
(the reaction can
only reverse)
COH
..
..
COH
..
..
A tertiary
amine
Protonated
carbonyl
C
..
OH
+
C
..
..
OH
+
+
R
R
R
N
R
3
N
..
FIGURE 16.52 Tertiary amines (R
3
N)
can add to carbonyl groups, but the
only available further reaction is
reversion to starting materials.
PROBLEM 16.18 Write a mechanism for the general reaction of Figure 16.50, the
acid-catalyzed reaction of a carbonyl compound with RNH
2
.
Note also that the success of the imine-forming reaction relies on the presence
of at least two hydrogens in the starting amine. One is lost as a proton in the first
step of the reaction, the formation of the carbinolamine. The second proton is lost
in a deprotonation step as the final imine is produced (Fig. 16.51).
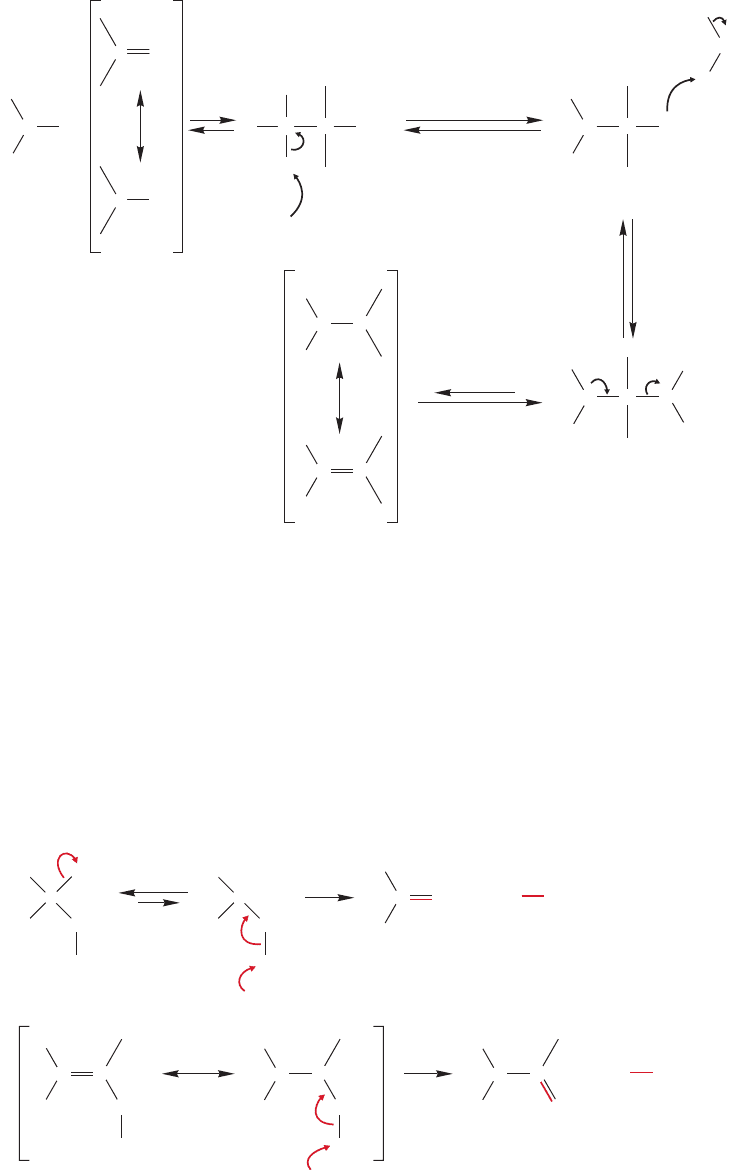
Secondary amines (R
2
NH) have one hydrogen, and so carbinolamine formation
is possible (Fig. 16.53).This reaction can be followed by protonation and water loss
to give a resonance-stabilized cation, called an iminium ion. An iminium ion has
the general structure . Deprotonation of the iminium ion on nitrogen
is not possible as there remains no second proton on nitrogen to be lost.
R
2
N
+
P
CR
2
16.11 Addition Reactions of Nitrogen Bases: Imine and Enamine Formation 795
first (and only)
deprotonation
An iminium ion (no proton can be lost from nitrogen
to give an imine because there is no H left attached to N)
..
N
R
R
..
N
R
R
..
N
R
R
H
NR
2
H
H
+
COH
..
..
C COH
..
..
..
R
..
..
..
C
C
N
R
R
H
2
OR
2
NH
N
R
R
+
+
C
..
OH
+
C
..
..
OH
+
R
..
+
H
H
O
R
2
NH
N
C
C
OH
..
..
OH
..
..
Carbinolamine
protonation
of oxygen
loss of
water
Secondary
amine
H
+
++
FIGURE 16.53 For secondary amines
(R
2
NH) carbinolamine formation is
possible, then water is lost to give a
resonance-stabilized iminium ion.There
is no hydrogen remaining on nitrogen
that can be lost to give an imine.
Think now about what you know of the possibilities for proton loss by carbo-
cations. The E1 reaction (p. 298) is a perfect example. In the E1 reaction, a leav-
ing group departs to give a carbocation, which reacts further by deprotonating at
all possible adjacent positions. If an adjacent carbon-attached hydrogen is avail-
able, the iminium ion can do this same reaction. Figure 16.54 makes the analogy.
This reaction gives a molecule called an enamine, which is the final product of
the reaction of secondary amines and simple aldehydes and ketones. Enamine is
the name used to describe a compound that has an alkene attached to an amine.
C
N
R
R
+
CH
2
C
R
R
..
C
N
R
R
+
..
C
N
R
R
Base
X
CH
2
C
R
R
E
1
H
CH
2
H
CH
2
H
CH
2
C
R
R
H
+
..
Base
..
Alkene
H
B
Enamine
CH
2
+
H
B+
FIGURE 16.54 Deprotonation of an
iminium ion to give an enamine is
exactly like deprotonation of a
carbocation to give an alkene.
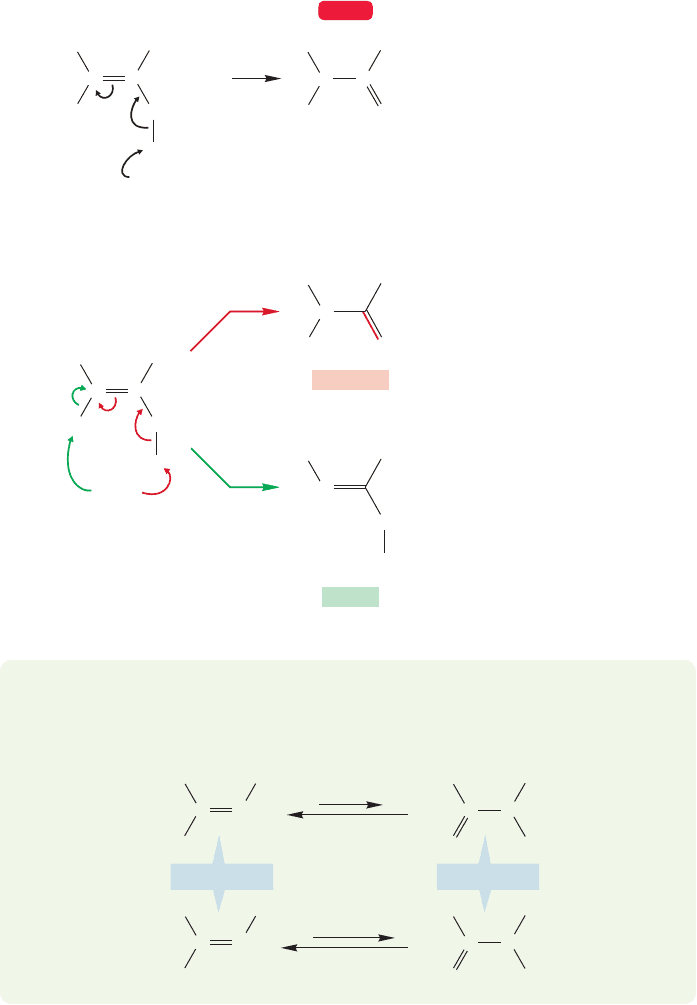
796 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
Notice that enamines are the nitrogen counterpart of enols (p. 448). Now comes an
important question: Why doesn’t the iminium ion formed from primary amines or
ammonia (Fig. 16.49) also lose a proton from carbon to give an enamine? Why is
imine formation preferred when there is a choice (Fig. 16.55)? The answer is extraor-
dinarily simple: Just as the ketone tautomer is more stable than the enol, the imine
is more stable than the enamine and so is preferred when possible. It is only in cases
where imine formation is not possible, as when secondary amines are used, that
enamines are formed. We hope you have noticed that the imine- and enamine-
forming reactions are reversible. The reactions work best under slightly acidic con-
ditions with heat applied to drive off water. If water is added to an imine or enamine,
the reverse reaction occurs to yield the carbonyl compound and an amine.
C
N
R
R
+
CH
2
H
..
C
N
R
R
CH
2
Base
CH
3
CH
3
..
Iminium ion from
a secondary amine
No choice here—base can
only remove H
+
from C;
there are no H atoms on N
C
N
R
H
+
CH
2
H
H
..
N
R
H
CH
2
Base
CH
3
CH
3
..
N
R
CH
2
CH
3
..
Iminium ion from
a primary amine
Here there is a choice;
base can remove H
+
from
either C (red) or N (green)
Enamine
(less stable)
Enamine
Imine
(more stable)
or
WEB 3D
FIGURE 16.55 Why don’t iminium
ions formed from primary amines
give enamines rather than imines?
PROBLEM 16.19 Many enamines and imines are in equilibrium. Write a mecha-
nism for the forward and the reverse pathways in the acid- and base-catalyzed
reactions below.
R
N
C
H
3
C
H
3
C
Imine
RNH
3
+
..
R
H
N
C
H
3
C
H
2
C
..
RNH
2
R
RNH
N
C
H
3
C
H
3
C
..
R
H
N
C
H
3
C
H
2
C
..
RNH
2
Enamine
–
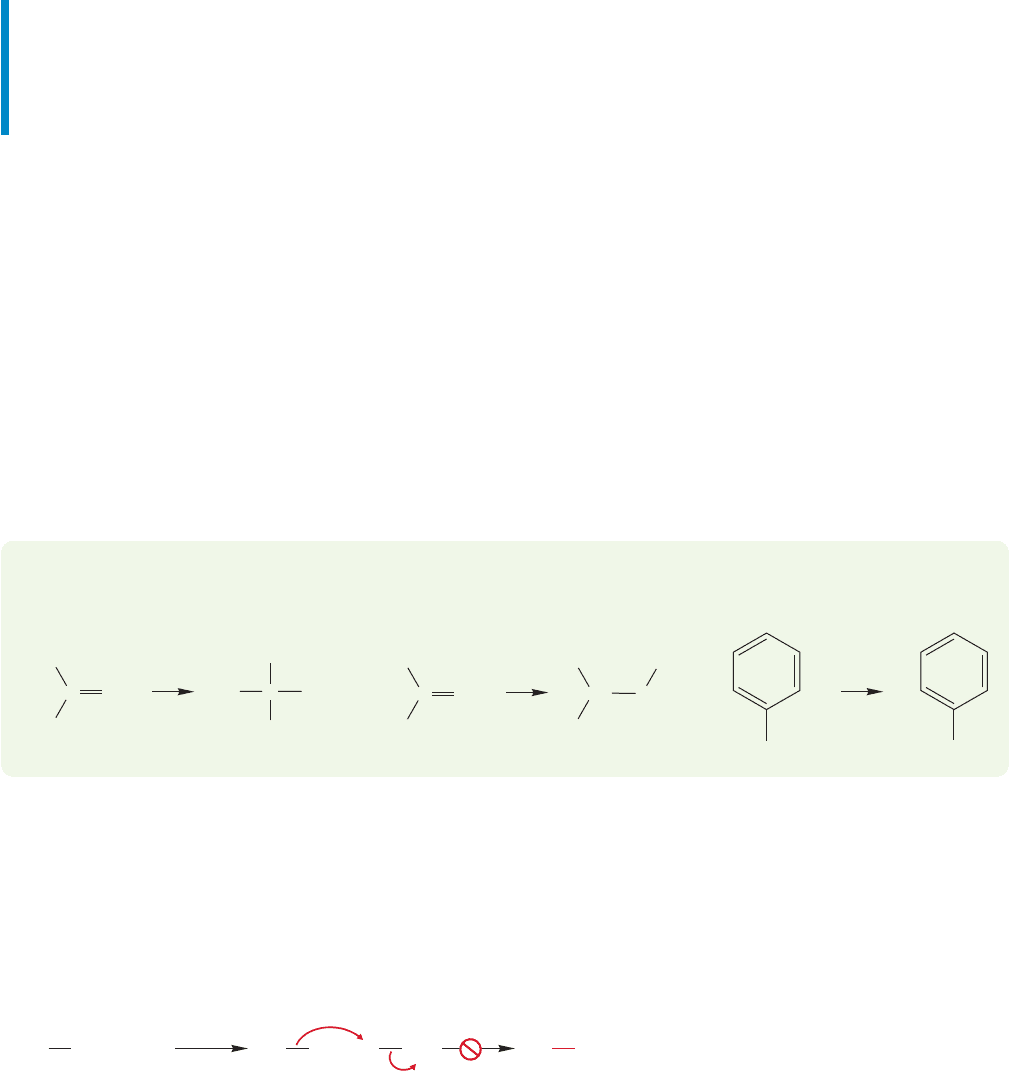
16.12 Organometallic Reagents 797
Summary
All manner of oxygen and nitrogen bases will add reversibly to carbonyl com-
pounds. The ultimate fate of the reaction and the ultimate structure of the
product depend on concentration, and, for nitrogen bases, on the number of
hydrogens available on nitrogen.
16.12 Organometallic Reagents
Not all additions to carbonyl compounds are reversible. Some of the irreversible reac-
tions are of great importance in synthetic chemistry, as they involve the creation of
carbon–carbon bonds. In order to discuss these, we must first talk a bit about the
formation of organometallic reagents.
We first met organometallic reagents in Section 6.3 (p. 227). It would be a very
good idea to go back and re-read those few pages carefully. You will recall that we
can make Grignard reagents (RMgX) and organolithium reagents (RLi) from the
corresponding alkyl halides. We observed that these reagents will react with water
or D
2
O to produce or , respectively (p. 229). We have also noted that
organometallic compounds add to epoxides (p. 429).
R
O
DR
O
H
PROBLEM 16.20 Given inorganic reagents of your choice (including D
2
O), devise
syntheses of the following molecules from the indicated starting materials:
CH
2
CH
3
CH
3
C
C
H
3
C
H
3
C
H
3
C
D
D
CH
2
C
H
3
C
H
3
C
CH
2
H
3
C
H
3
C
NO
2
D
CH
Neither Grignard reagents nor organolithium reagents are good nucleophiles
toward organic halides (Fig. 16.56). If they were, the syntheses we use to generate
the organometallic reagents in the presence of the starting organic halide would be
quite ineffective,because hydrocarbons would be the major products.Small amounts
of coupling are sometimes observed, especially with highly reactive halides such as
allyl bromides.
RX RLiLi RX RR
LiX
++
FIGURE 16.56 Organometallic reagents are strong bases but not strong nucleophiles.
There are organometallic reagents that can be used to displace halides to good
effect. The most common examples are the organocuprates formed from organo-
lithium compounds and copper iodide. The organocuprates are quite versatile
reagents, and undergo a variety of synthetically useful reactions. We will meet them
again a number of times, but for now, note that they are able to displace chloride,
bromide, and iodide ions, as well as other good leaving groups, from primary alkyl
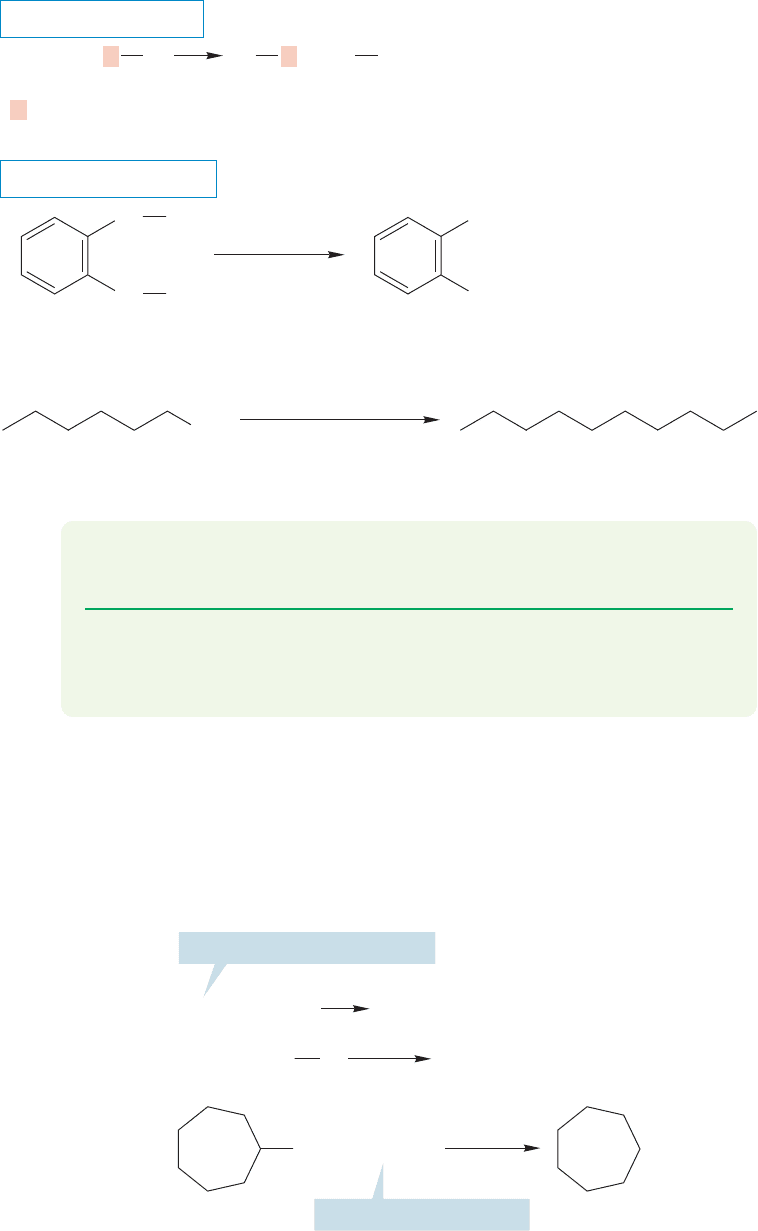
798 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
halides and often from secondary alkyl halides (Fig.16.57).This capability gives you
another quite effective synthesis of hydrocarbons.
X = I, Cl, Br, or other good leaving groups
R = Primary or secondary alkyl group
OTs = tosylate, an excellent leaving group
(77%)
(98%)
0 ⬚C, ether
RR
2
CuLi X
excess
(CH
3
)
2
CuLi
–75 ⬚C, ether
(CH
3
CH
2
CH
2
CH
2
)
2
CuLi
OTs
+
R
LiX
R
++
RCu
CH
2
Cl
CH
2
Cl
CH
2
CH
3
CH
2
CH
3
THE GENERAL CASE
SPECIFIC EXAMPLES
FIGURE 16.57 Lithium
organocuprates will react
with primary and
secondary alkyl halides to
give hydrocarbons.
Many metal hydrides,such as lithium aluminum hydride (LiAlH
4
),undergo reac-
tions that are similar to those of organometallic reagents. Many hydride reagents react
violently with moisture to liberate hydrogen gas. Lithium aluminum hydride and
some other metal hydrides are strong enough nucleophiles to displace halides from
most organic halides.Lithium triethylborohydride,LiHB(Et)
3
,is an especially effec-
tive H:
source that can be used as a displacing agent (Fig. 16.58).
LiH
2
+
HOAlH
3
Lithium aluminum hydride (LAH)
Lithium triethylborohydride
+
H
2
OLiAlH
4
+
–
+
+
CH
4
LiAlH
4
ether
LiHB(Et)
3
CH
3
I
Br
THF
(100%)
(99%)
25 ⬚C
FIGURE 16.58 Metal hydrides react
with water to generate hydrogen, and
are often strong enough nucleophiles
to displace halides and generate
hydrocarbons.
PROBLEM 16.21 Why is tosylate such a good leaving group? If you can’t recall the
structure of tosylate—and you need that first—see page 283.
PROBLEM 16.22 One possible mechanism for hydrocarbon formation by
organocuprates is an S
N
2 displacement by one of the R groups on the alkyl halide.
Can you devise an experiment to test this possibility?
