Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

16.13 Irreversible Addition Reactions: A General Synthesis of Alcohols 799
Like other Lewis bases, Grignard reagents, organolithium reagents, and metal
hydrides are also able to undergo addition to carbonyl-containing compounds.These
additions lead directly to the next subject.
16.13 Irreversible Addition Reactions: A General
Synthesis of Alcohols
Now that we know something of the structures of these organometallic reagents,
it is time to look at their reactivity with ketones and aldehydes. This subject is
important, because these reactions form carbon–carbon bonds, a central goal in
synthetic chemistry. Like oxygen- and nitrogen-centered nucleophiles, organometallic
reagents (and metal hydrides) add to carbonyl compounds. Although there is no free
R
(or H
) in these reactions, the organometallic reagents are reactive enough to
deliver an R
group, acting as a nucleophile, to the Lewis acidic carbon of the car-
bonyl group (Fig. 16.59). The initial product is a metal alkoxide, which is protonated
:
::
O
C
..
Addition
Protonation
Li
R
C
..
..
R
Li
+
O
..
O
C
..
..
..
MgX
R
C
..
..
R
MgX
O
..
Grignard
reagents
Organolithium
reagents
Metal alkoxides
Alcohols
Metal alkoxides
C
..
..
R
Li
+
O
..
C
..
..
R
MgX
O
..
C
..
..
R
LiOH
OH
C
..
..
R
HOMgX
OH
+
+
..
..
H
2
O
H
3
O
..
+
..
..
H
2
O
H
3
O
..
+
Alcohols
–
–
–
+
+
–
Addition
Protonation
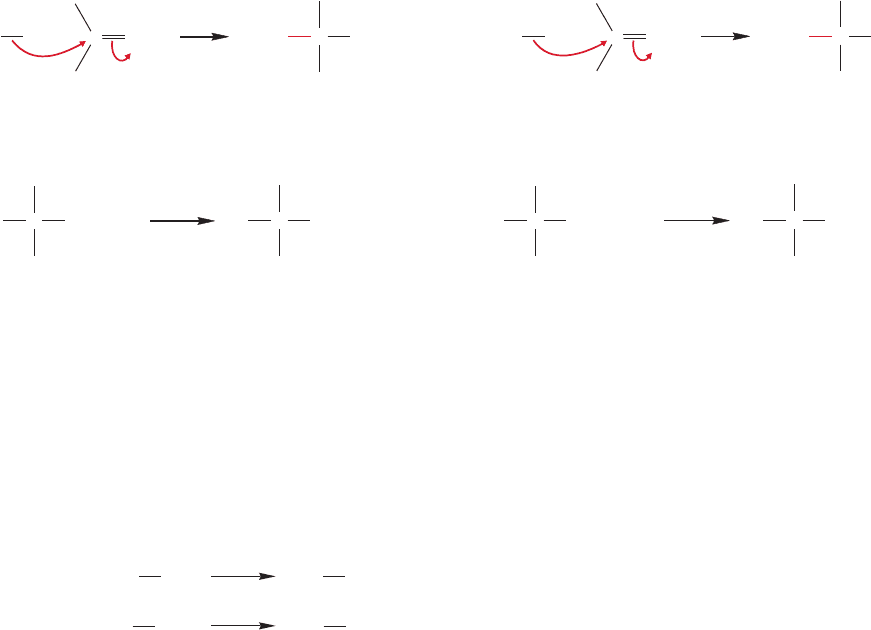
FIGURE 16.59 Organometallic reagents are strong enough nucleophiles to add to carbonyl
compounds. When water is added in a second step, alcohols are produced.
when the solution is neutralized by addition of aqueous acid in a second step.Water
cannot be present at the start of the reaction because it would destroy the
organometallic reagent (Fig. 16.60).
R
Li
R
MgX
H
R
LiOH
H
R
HOMgX
+
+
..
..
H
2
O
..
..
H
2
O
FIGURE 16.60 If water is present at
the beginning of the reaction, the
organometallic reagent is destroyed
through hydrocarbon formation.
It is easy to make the mistake of writing this reaction sequence as shown in Figure
16.61a, which means, “Treat the ketone with a mixture of alkyllithium reagent and
water.”This procedure is impossible, because the water would destroy the alkyllithi-
um immediately (Fig. 16.60). The correct way to write the sequence is shown in
800 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
Figure 16.61b and means, “First allow the ketone to react with the alkyllithium
reagent and then, after some specified time, add water.”
..
..
..
..
RMgX
+
..
H
2
O
+
H
H
C
O
O
H
H
C
..
..
R
CH
2
OH
A primary
alcohol
..
..
..
..
..
..
MgX
..
..
..
H
2
O
+
H
R
O
O
H
R
C
..
..
..
..
H
2
O
OH
..
or
..
..
..
+
O
H
2
C
1. RMgX
2. H
2
O
RCH
2
OH
R
C
RMgX
H
R
C
R
..
..
..
..
MgX
..
+
H
R
O
O
H
R
C
..
..
OH
R
C
RMgX
H
R
C
R
+
Secondary alcohols
..
..
O
H
MgBr
2.
1.
..
..
OH
(60%)
RMgX
–
..
..
–
–
H
3
O
..
+
..
..
O
2.
1.
..
..
(69%)
H
3
O
..
+
GENERAL CASES
SPECIFIC EXAMPLES
H
2
C
MgCl
OH
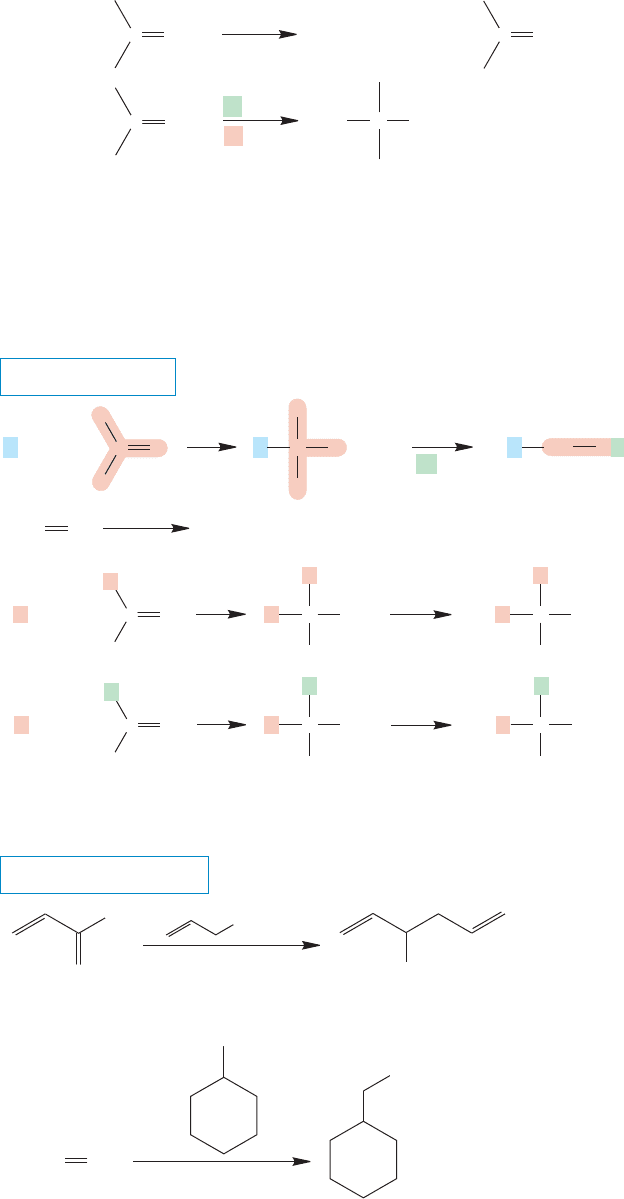
FIGURE 16.62 Reaction of
organometallic reagents with
formaldehyde, followed by hydrolysis,
leads to primary alcohols.The
reaction of other aldehydes with
organometallic reagents, followed by
hydrolysis, gives secondary alcohols.
OC
..
OC
..
RH
O
C
..
..
..
C
..
..
R
OH
LiOH+
+
..
..
H
2
O
..
..
H
2
O
RLi
RLi
..
..
..
1.
2.
(a)
(b)
FIGURE 16.61 Reaction (a) means,
Add a mixture of and water
to the carbonyl compound. Under
such conditions the organometallic
reagent will be destroyed. Reaction
(b) means, First add the
organometallic reagent to the
carbonyl compound and then, in a
second step, add water.
R
O
Li
This addition of organometallic to carbonyl compounds gives us a new and most
versatile alcohol synthesis. Primary alcohols can be made through the reaction of an
organometallic reagent with formaldehyde (Fig. 16.62). Figure 16.62 also shows the
shorthand form of the reaction, which emphasizes the synthetic utility.
16.13 Irreversible Addition Reactions: A General Synthesis of Alcohols 801
Metal hydrides (LiAlH
4
, NaBH
4
, and many others) react in a similar fashion to
deliver hydride (H
) as the nucleophile (Fig. 16.64). Alcohols are formed when
water is added in a second, quenching step. Notice that this reaction is formally a
reduction. Aldehydes are reduced to primary alcohols and ketones are reduced to
secondary alcohols.There is no way to make a tertiary alcohol through this kind of
reduction (Fig. 16.64).
:
Similarly, reactions of organometallic reagents (RLi or RMgX) with alde-
hydes other than formaldehyde give secondary alcohols. The R of the organo-
metallic reagent can either be the same as that of the aldehyde or different
(Fig. 16.62).
Ketones react with Grignard reagents or organolithium reagents to give tertiary
alcohols. Three different substitution patterns are possible depending on the num-
ber of different R groups in the reaction (Fig. 16.63).
A SPECIFIC EXAMPLE
2. 0 ⬚C, H
2
O
..
..
OH
..
..
O
1. CH
3
MgBr
ether
CH
3
(95%)
..
..
MgX
+
..
..
..
..
+
R
O
O
R
C
..
..
OH
R
C
RMgX
(or RLi)
RMgX
(or RLi)
R
C
R
R
RR R
MgX
+
..
..
..
+
R
O
O
R
C
..
..
OH
R
C
R
C
R
R
RR R
MgX
+
..
..
..
..
..
+
R
O
O
R
C
..
..
OH
R
C
RMgX
(or RLi)
RMgX
(or RLi)
R
C
R
R
RR R
MgX
+
..
..
..
..
+
R
O
O
R
C
..
..
OH
R
C
R
C
R
R
RR R
–
..
..
–
..
–
..
–
..
..
H
2
O
..
..
H
2
O
..
..
H
2
O
..
..
H
2
O
THE GENERAL CASE
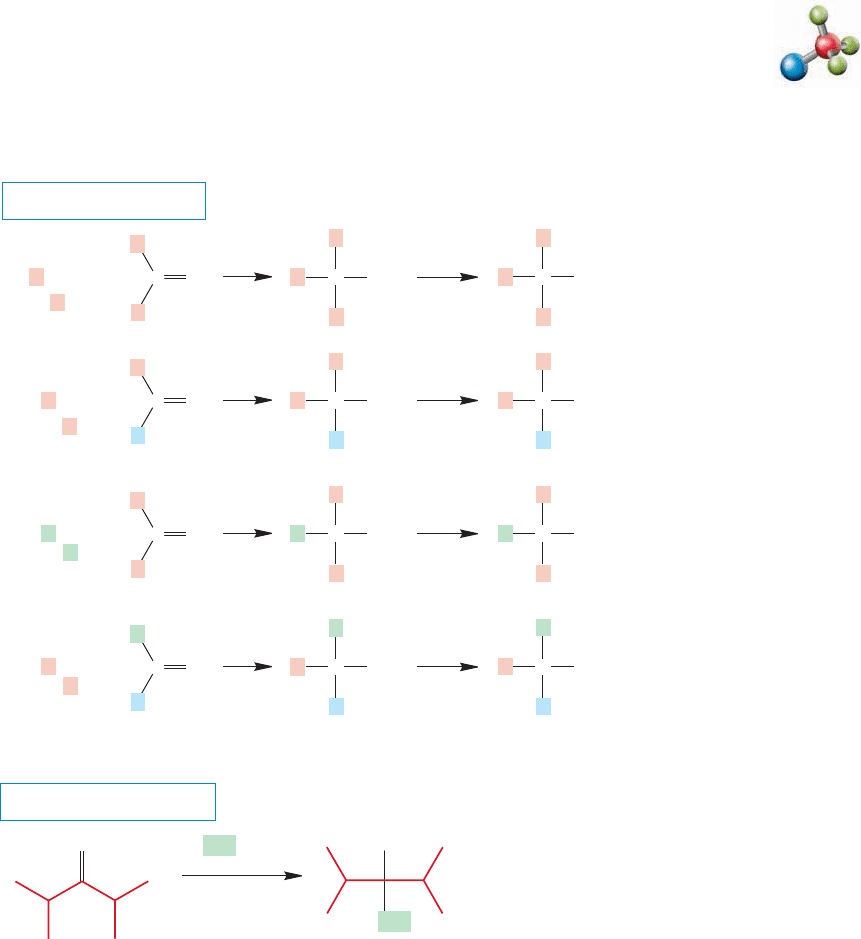
FIGURE 16.63 The reaction of ketones with organometallic reagents, followed
by hydrolysis, yields tertiary alcohols.
Grignard reaction
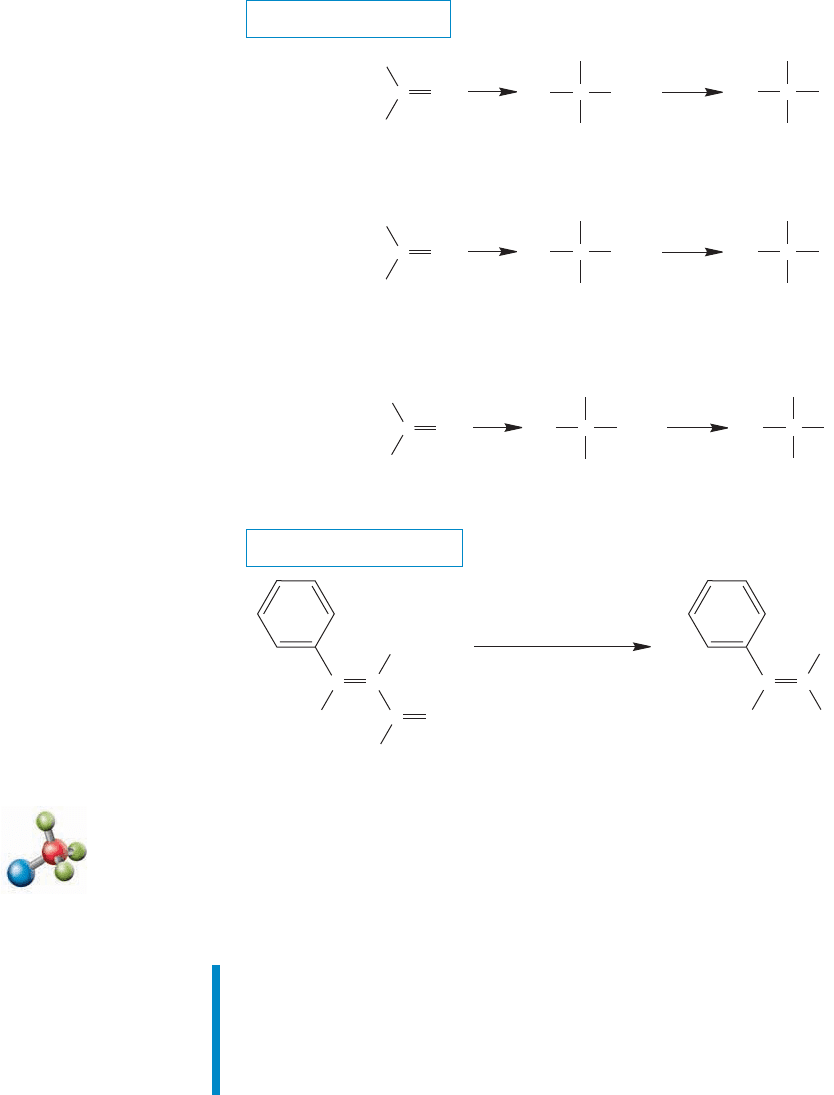
We have seen reduction of carbonyl compounds before. In Chapter 14 (p. 645),
we encountered the Clemmensen and Wolff–Kishner reductions in which a
carbon–oxygen double bond is reduced all the way to a methylene group.
802 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
Li
+
CH
3
OH, 20–30 ⬚C
..
..
..
..
..
H
2
O
+
O
O
–
–
–
H
C
..
..
OH
H
C
LiAlH
4
(or NaBH
4
)
H
C
H
HH
Li
+
..
..
..
..
..
H
2
O
+
O
O
R
C
..
..
OH
H
C
LiAlH
4
(or NaBH
4
)
R
C
H
HH
Li
+
..
..
..
..
..
H
2
O
+
O
O
R
C
..
..
OH
H
C
LiAlH
4
(or NaBH
4
)
R
C
H
RRR
1. NaBH
4
(97%)
H
H
R
H
R
R
..
..
..
..
..
..
Methyl alcoholFormaldehyde
A primary
alcohol
A secondary
alcohol
CC
H
H
C
H
..
..
O
2. H
2
O
..
..
CC
H
..
..
CH
2
OH
H
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 16.64 Metal hydride
addition, followed by hydrolysis, gives
primary alcohols from aldehydes and
secondary alcohols from ketones.
Carbonyl reduction
Summary
The irreversible addition of organometallic reagents and metal hydrides provides
a simple synthesis of primary, secondary, and tertiary alcohols. But you have to
be careful to choose your reagents properly, so that the desired substitution pat-
tern is achieved.
16.14 Oxidation of Alcohols to Carbonyl Compounds
16.14a Oxidation of Monoalcohols So far, we have generated two new and
very general alcohol syntheses, the reaction of aldehydes and ketones with
organometallic reagents, and the reduction of carbonyl compounds by metal
hydrides. A complement to the reduction reaction is the oxidation of alcohols to
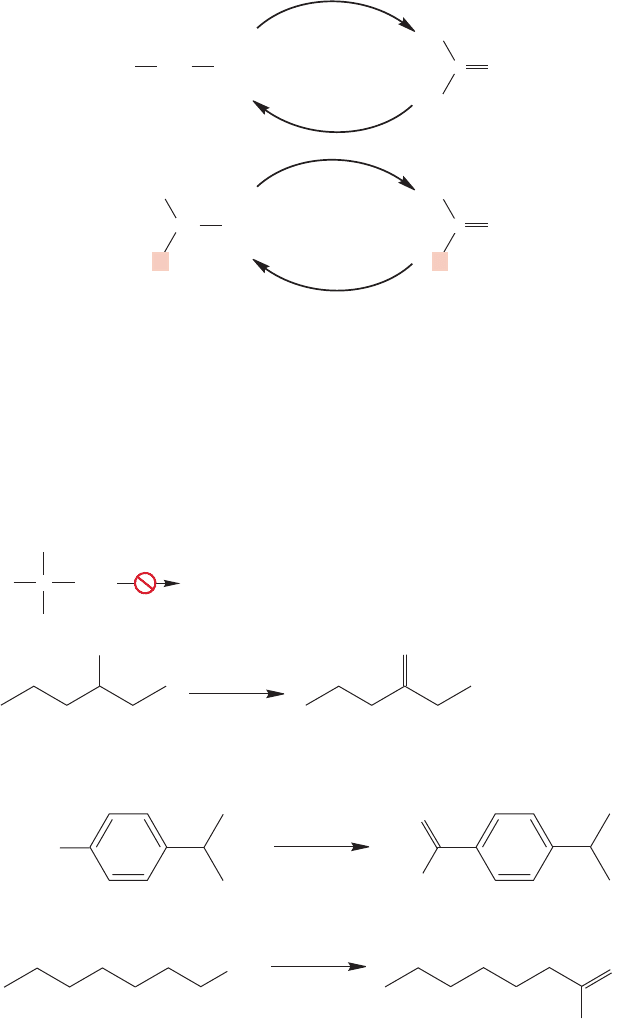
aldehydes and ketones (Fig. 16.65). These oxidations extend the utility of the alco-
hol syntheses we have just learned, and complicate your life by increasing the
complexity of the molecules you are able to make.
16.14 Oxidation of Alcohols to Carbonyl Compounds 803
..
O
C
..
..
OH
CH
2
R
R
H
oxidation
reduction
..
..
..
O
C
..
..
OH
R
oxidation
reduction
R
R
R
Reduction conditions: 1. LiAlH
4
(or NaBH
4
)
2. H
2
O
CH
FIGURE 16.65 Alcohols can be
oxidized to carbonyl compounds.
This reaction is the reverse of what
you have just learned—the reduction
of carbonyl compounds to alcohols
through reactions with metal hydride
reagents.
No simple oxidation
products
OH
OH
R
C
R
R
OH O
H
2
SO
4
K
2
Cr
2
O
7
3-Hexanone
(80%)
HOCH
2
CrO
3
pyridine
4-Isopropylbenzaldehyde
(83%)
H
2
O
H
2
CrO
4
O
OH
Heptanoic acid
(70%)
O
H
FIGURE 16.66 Tertiary alcohols
cannot be easily oxidized, but
secondary and primary alcohols can
be. It is logical to infer that in order
for the oxidation reaction to succeed
there must be a carbon–hydrogen
bond available on the alcohol carbon.
Now your collection of syntheses can be extended by using the alcohols as starting
materials in making carbonyl compounds using the oxidation reaction. Tertiary alco-
hols cannot be easily oxidized, but primary and secondary ones can be. As shown in
Figure 16.66, secondary alcohols can only give ketones, but primary alcohols can give
either aldehydes or carboxylic acids (RCOOH) depending on the oxidizing agent used.
How do these oxidation reactions work? A typical oxidizing agent for primary alco-
hols is chromium trioxide (CrO
3
) in pyridine. As long as the reagent is kept dry, good
yields of aldehydes can often be obtained (Fig. 16.66). Although a metal–oxygen bond
is more complicated than a carbon–oxygen bond, the double bonds in CrO
3
and
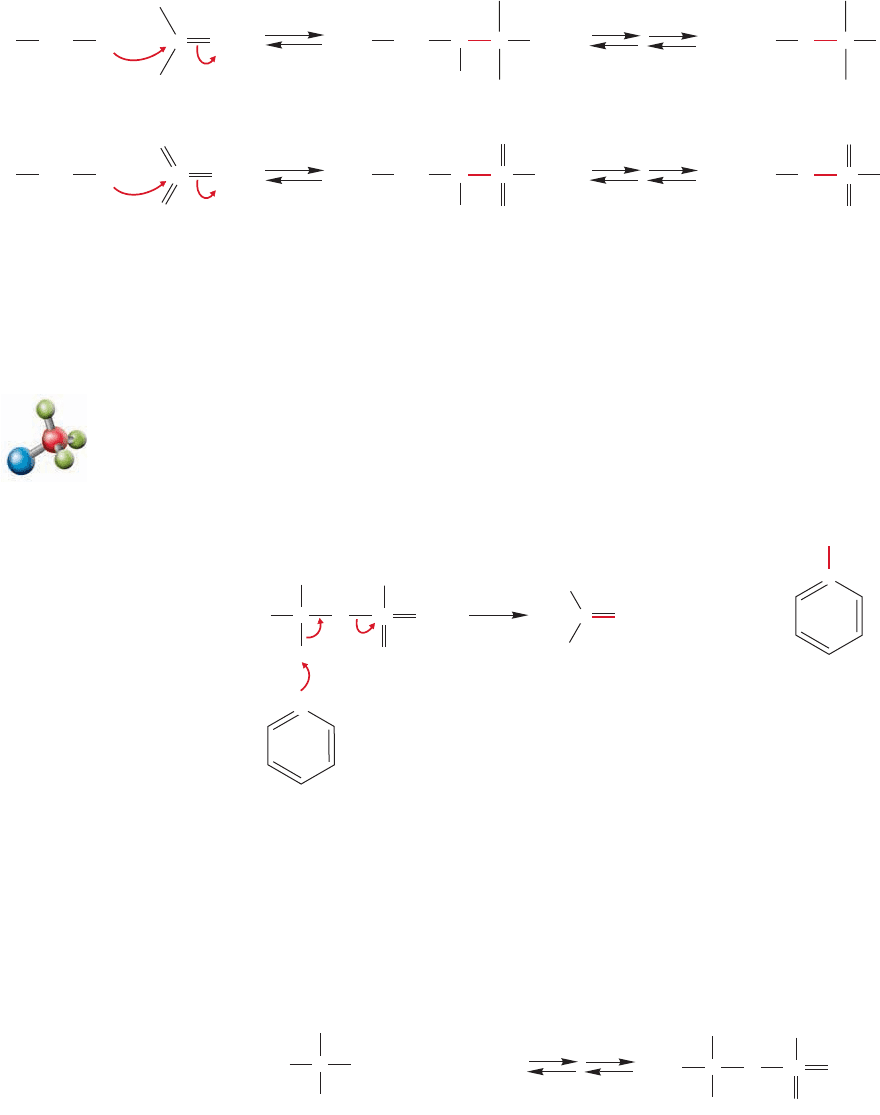
804 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
react in similar ways. For example, an alcohol oxygen is a nucleophile and
can attack the chromium–oxygen bond to give, ultimately, a chromate ester inter-
mediate (Fig. 16.67). This reaction of a with an alcohol is analogous to the
formation of a hemiacetal from the reaction of a with an alcohol.C
P
O
Cr
P
O
R
2
C
P
O
O
C
..
..
O
..
..
C
..
..
O
proton
transfers
proton
transfers
..
..
..
..
..
OH
CH
2
R
..
CH
2
R
O
H
C
..
..
OH
..
..
RCH
2
O
Hemiacetal
Cr
..
..
O
..
..
..
OH
CH
2
R
..
CH
2
R
O
H
..
..
OH
..
..
RCH
2
O
A chromate ester
intermediate
O
..
O
..
O
..
..
O
..
..
Cr
O
..
..
O
..
..
Cr
+
–
+
–
FIGURE 16.67 Nucleophilic alcohols can add to bonds just as they do to bonds.
This reaction is the first step in the oxidation process, and in this case gives a chromate ester
intermediate.
C
P
OCr
P
O
Once the chromate intermediate is formed, pyridine (or any other base) initi-
ates an E2 reaction to give an aldehyde (an oxidized alcohol) and HOCrO
2
(a reduced chromium species) (Fig. 16.68).
N
O
C
..
..
C
..
R
H
..
..
O
..
..
O
R
H
Cr
OH
..
O
Pyridine
..
..
H
..
E2
HOCrO
2
+
N
+
+
H
–
FIGURE 16.68 The second step in the
oxidation reaction is a simple E2
reaction to generate the new
double bond.
C
P
O
No H available
for an E2 reaction!
CrO
3
+
C
..
R
R
..
..
O
..
..
O
Cr
OH
..
O
..
..
R
C
R
R
..
..
OH
R
FIGURE 16.69 A tertiary alcohol can
add to the double bond, but
there is no hydrogen in the chromate
intermediate available for an E2
reaction.
Cr
P
O
Chromium trioxide (CrO
3
) will also oxidize secondary alcohols to ketones. It
should now be clear why tertiary alcohols resist oxidation by this reagent. The ini-
tial addition reaction can proceed, but there is no hydrogen to be removed in the
elimination process (Fig. 16.69).
Alcohol oxidation
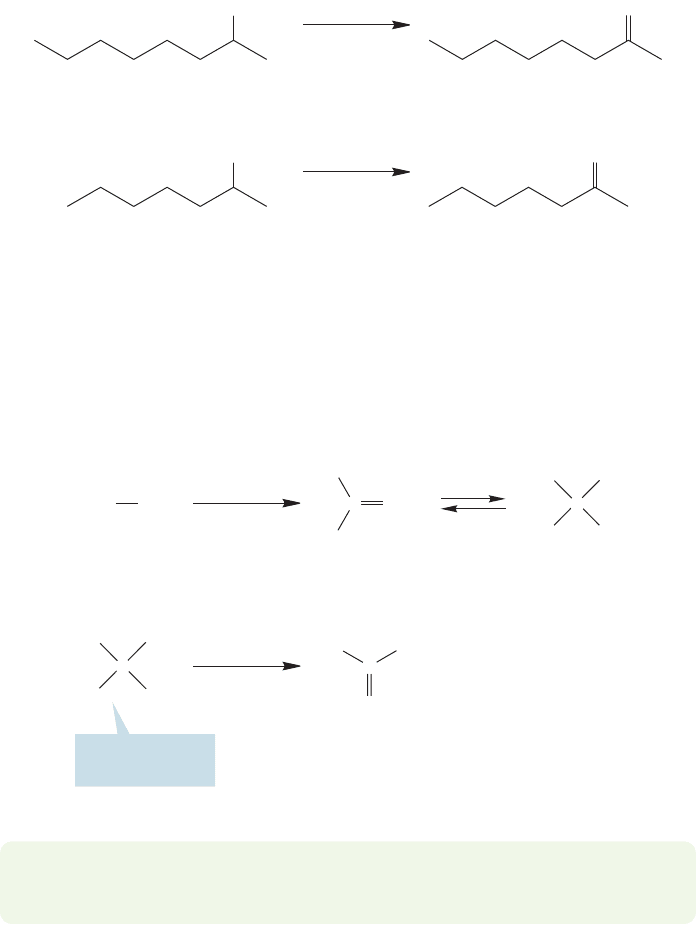
16.14 Oxidation of Alcohols to Carbonyl Compounds 805
100 ⬚C, 2 h
H
2
SO
4
Na
2
Cr
2
O
7
CH
3
COOH
< 30 ⬚C
CrO
3
OH
O
(94%)
OH
(83%)
O
FIGURE 16.70 A typical reagent for
oxidation of secondary alcohols to
ketones is sodium dichromate in acid.
Other oxidizing agents also work.
Notice how this seemingly strange reaction, oxidation of alcohols with CrO
3
,
is really made up of two processes we have already encountered. The first step is
addition of an oxygen base to a chromium–oxygen double bond. The second step
is nothing more than a garden-variety elimination. The lesson here is that you
should not be bothered by the identity of the atoms involved.When you see a reac-
tion that looks strange, rely on what you already know. Make analogies, and more
often than not you will come out all right.That is exactly what all chemists do when
we see a new reaction. We think first about related reactions and try to find a way
to generalize.
Another useful oxidizing reagent is sodium chromate (Na
2
CrO
4
) or sodium
dichromate (Na
2
Cr
2
O
7
) in aqueous strong acid (Fig. 16.70). Under these aqueous
conditions, it is difficult to stop oxidation of primary alcohols at the aldehyde stage.
C
..
.. ..
..
....
O
OH
RCH
2
C
R
H
Aldehyde
intermediate
H
2
SO
4
H
2
O
Na
2
CrO
4
H
2
SO
4
H
2
O
Na
2
CrO
4
H
2
O
..
R
H
..
OH
OH
Hydrated
aldehyde
C
..
R
H
..
OH
OH
A carboxylic
acid
OH
C
R
..
..
..
..
.. ..
O
This hydrate
can be oxidized
FIGURE 16.71 Oxidation of a
primary alcohol leads to an aldehyde.
Aldehydes are hydrated in the
presence of water, and the hydrates
are alcohols. Further oxidation of the
hydrate leads to the carboxylic acid.
PROBLEM 16.23 Write a mechanism for the oxidation of a secondary alcohol to a
ketone by chromic oxide.
Overoxidation to the carboxylic acid almost always occurs. The difficulty is that
aldehydes are hydrated in water (p. 773). These hydrates are alcohols and can be
oxidized further (Fig. 16.71). If one wants to convert a primary alcohol into a car-
boxylic acid, Na
2
Cr
2
O
7
in H
2
SO
4
and H
2
O is the reagent of choice.
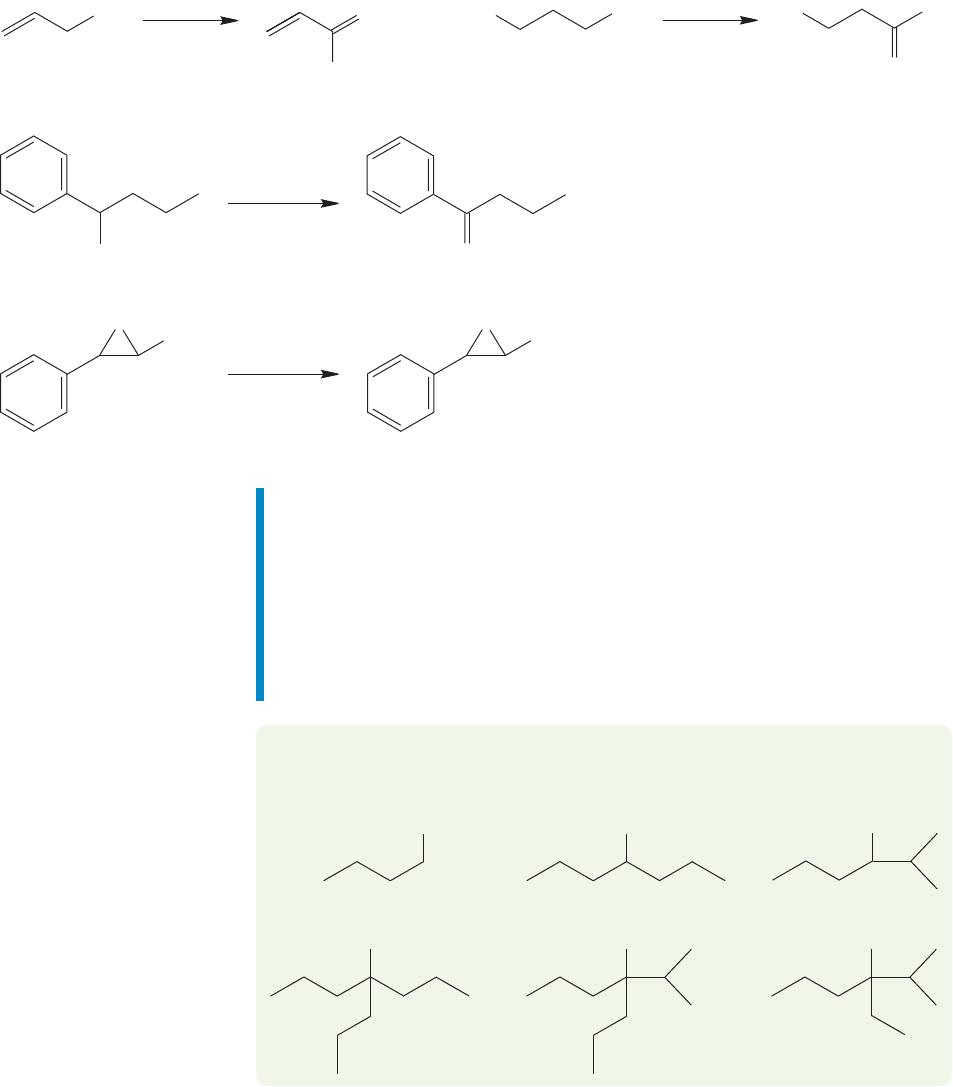
806 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
MnO
2
OH
pentane
H
(60%)
(79%)
(90%)
OH
OH
Cl
HNO
3
100 ⬚C
Cl
OH
KMnO
4
CH
3
COOH
25 ⬚C
O
O
(87%)
DMSO
(COCl)
2
O
O
CH
2
OH
O
CHO
FIGURE 16.72 Some oxidizing agents.
In addition to the chromium reagents, there are many other oxidizing agents of
varying strength and selectivity (Fig. 16.72). Manganese dioxide (MnO
2
) is effec-
tive at making aldehydes from allylic or benzylic alcohols, nitric acid (HNO
3
) oxi-
dizes primary alcohols to carboxylic acids, and potassium permanganate (KMnO
4
)
is an excellent oxidizer of secondary alcohols to ketones and of primary alcohols to
carboxylic acids, although it can be difficult to control. One of the mildest and most
useful oxidizing reagents is dimethyl sulfoxide (DMSO), which can be used in com-
bination with one of a variety of co-reactants to convert secondary alcohols into
ketones and primary alcohols into aldehydes.
Summary
Now we can reduce carbonyl compounds to the alcohol level with hydride reagents,
and oxidize them back again using the various reagents of this section. Although
one probably wouldn’t want to spend one’s life cycling a compound back and forth
between the alcohol and carbonyl stage, these reactions are enormously useful in
synthesis. Many alcohols are now the equivalent of aldehydes or ketones, because
they can be oxidized, and carbonyl compounds are the equivalent of alcohols because
they can be reduced with hydride or treated with an organometallic reagent.
PROBLEM 16.24 Starting from alcohols containing no more than three carbon atoms
and inorganic reagents of your choice, devise syntheses of the following molecules:
OH
OH
OH
(a) (b) (c)
OH OH OH
(d) (e) (f)
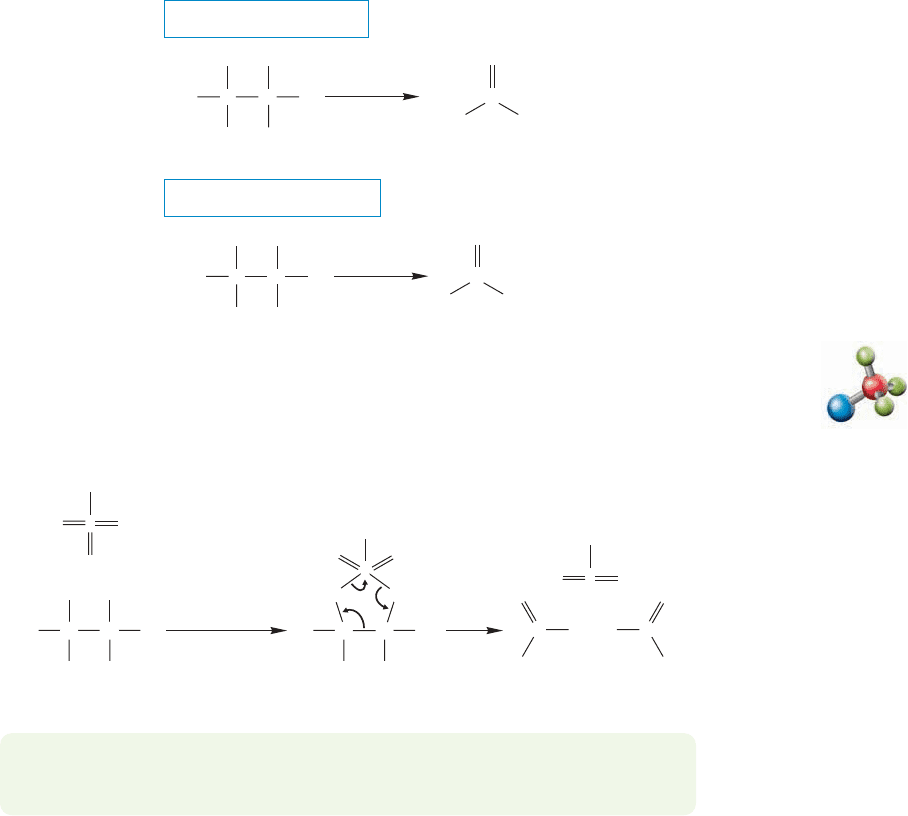
16.15 Retrosynthetic Alcohol Syntesis 807
16.14b Oxidative Cleavage of Vicinal Diols There is an oxidation reaction
that works only for vicinal diols (1,2-diols). When a vicinal diol is oxidized by per-
iodic acid (HIO
4
) the diol is cleaved to a pair of carbonyl compounds (Fig. 16.73).
This reaction, which has long been used as a test for 1,2-diols, involves the forma-
tion of a cyclic periodate intermediate.
THE GENERAL CASE
A SPECIFIC EXAMPLE
HIO
4
H
2
SO
4
R2R
HO
RR
RR
C
OH
CC
O
HIO
4
H
2
SO
4
HH
HO
HH
HH
C
OH
CC
(100%)
O
FIGURE 16.73 The cleavage of vicinal
diols by periodic acid.
C
O
O
OH
O
C
RR
RR
HIO
4
H
2
O/H
2
SO
4
Cyclic ester of periodic acid
I
O
OH
O
OO
I
R
HO
R
R
C
R
OH
OH
C
R
R
R
C
O O
OO
I
R
C
FIGURE 16.74 The vicinal diol
cleavage by periodic acid involves a
cyclic intermediate that breaks down
to give a pair of carbonyl compounds.
This reaction looks much like the formation of a cyclic acetal from a 1,2-diol
and a ketone.The intermediate breaks down to form the two carbonyl compounds.
As you can see from the mechanism (Fig. 16.74), only vicinal diols can undergo this
cleavage reaction.
PROBLEM 16.25 trans-1,2-Cyclohexanediol is much more reactive toward periodic
acid than the cis isomer. Explain why.
16.15 Retrosynthetic Alcohol Synthesis
It’s worth taking a moment to say a word again about strategy in doing synthe-
sis problems, and to reinforce an old idea. It is almost never a good idea to try to
see all the way back from the target molecule to the initial starting materials.
Chemists have learned to be much less ambitious and to work back one step at
a time. This process has been dignified with the title retrosynthetic analysis,
which makes the idea seem far more complicated than it is. Let’s assume you want
to make molecule A. The idea behind this retrosynthesis strategy is simply that
you can almost always see the synthetic step immediately leading to the product
A. That is, compound A could come from B. Sometimes the problem is one of
sorting among various possibilities of reactions that would lead to the product.
Each possibility might come from a different compound, so perhaps B, C, or D
are possible precursors to compound A. Now we just ask ourselves for a set of
Cleavage of vicinal diols
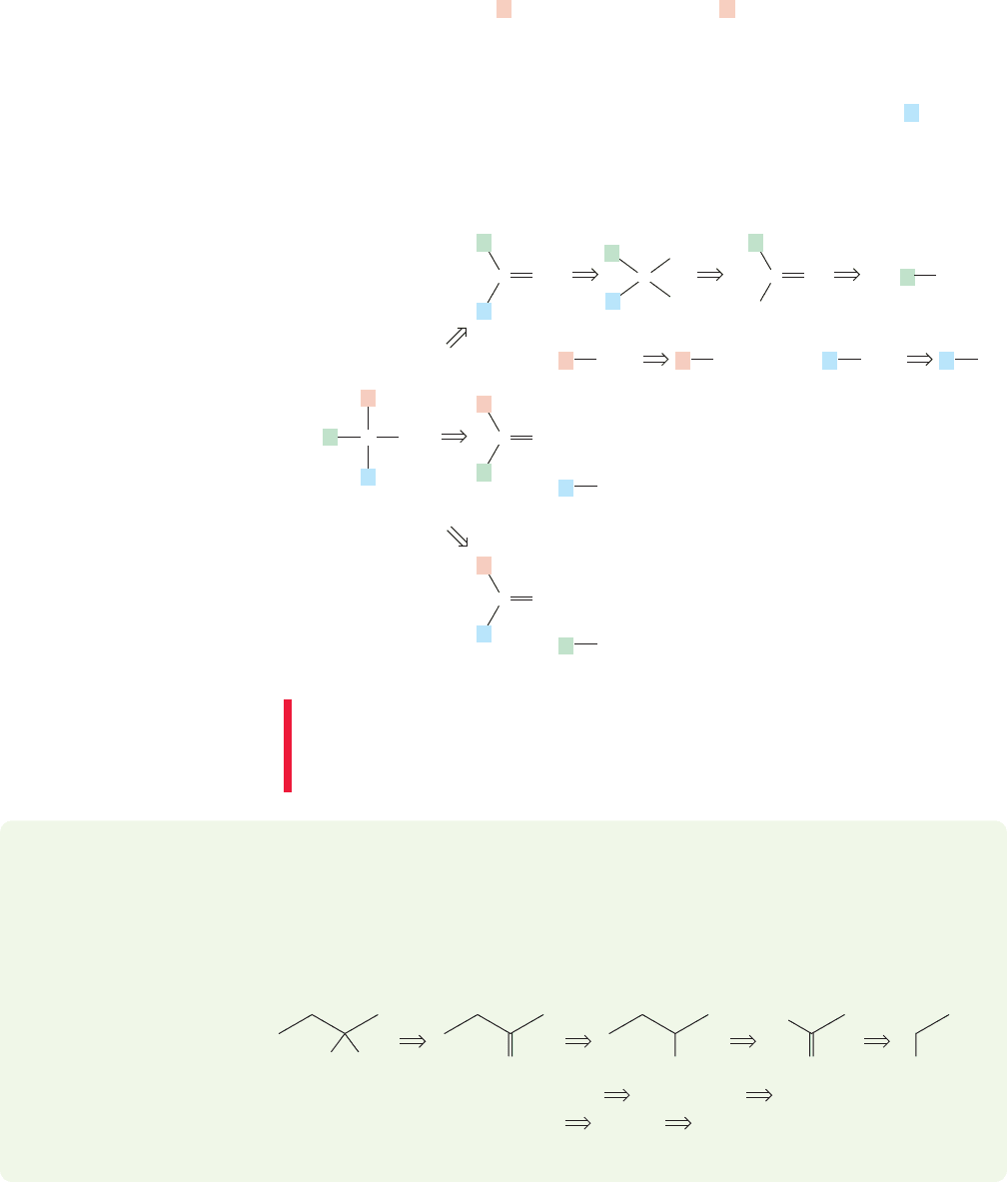
reactions leading to the new target B, or C, or D. The idea is that eventually this
technique will lead back to starting materials that are readily available. Don’t be
too proud to work back step by step, even in simple problems.
As an example of using retrosynthetic analysis, consider synthesizing a general
tertiary alcohol A (Fig. 16.75).It could be made in three ways.Your thoughts should
be led to each of the three ketones B, C, or D shown in the figure. You know the
retrosynthesis for the MgX. It comes from an X and Mg. To carry the analysis
further, you need to decide which ketone to use. Whichever one you choose, you
need to devise a synthesis for it. Fortunately, we have just covered this topic. You
know that a ketone can come from a secondary alcohol. So, B could be obtained
from the secondary alcohol E,which can come from an aldehyde F and MgX.The
beauty of it all is that aldehyde F comes from a primary alcohol G. So you will
inevitably find your way back to simple alcohols and alkyl halides in this analysis.
All that remains is to write out the forward reactions.
R
RR
808 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
..
..
OH
R
C
A
B
C
D
R
R
R
O
C
R
R
O
C
R
R
O
C
R
+
E
R
C
R
F
R
O
C
H
G
CH
2
OH
R
R
MgX
R
X
+
R
MgX
R
X
+
R
MgX
+
R
MgX
H
OH
FIGURE 16.75 A retrosynthetic
analysis of ways of making a tertiary
alcohol.
Note in Figure 16.75 that retrosynthetic analysis has appropriated a special arrow
for its own use. The double-lined arrow always points from the target molecule to
the immediate precursor. So a double-lined arrow pointing from A to B means that
A comes from B.
WORKED PROBLEM 16.26 Here is a specific example of using retrosynthetic analy-
sis. Provide a retrosynthetic synthesis of 2-phenyl-2-butanol.Assume that you have
benzene, ethanol, pyridine, and access to any inorganic reagents you might need.
ANSWER Here is the retrosynthetic analysis:
OHPh
O OOH OH
H
CH
3
CH
2
Li CH
3
CH
2
Br CH
3
CH
2
OH
PhLi
PhLi
PhBr
Li
+
CH
3
CH
2
Li
+
PhH
Br
2
/FeBr
3
CrO
3
/pyridine
CONVENTION ALERT
