Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

16.16 Oxidation of Thiols and Other Sulfur Compounds 809
(c)
(f)
OH
(b)
(e)
OH
Cl
OH
(a)
(d)
OTs
PROBLEM 16.27 Starting from inorganic reagents, tosyl chloride, formaldehyde,
acetaldehyde, acetone, and butyl alcohol, devise syntheses of the following
molecules:
16.16 Oxidation of Thiols and Other Sulfur
Compounds
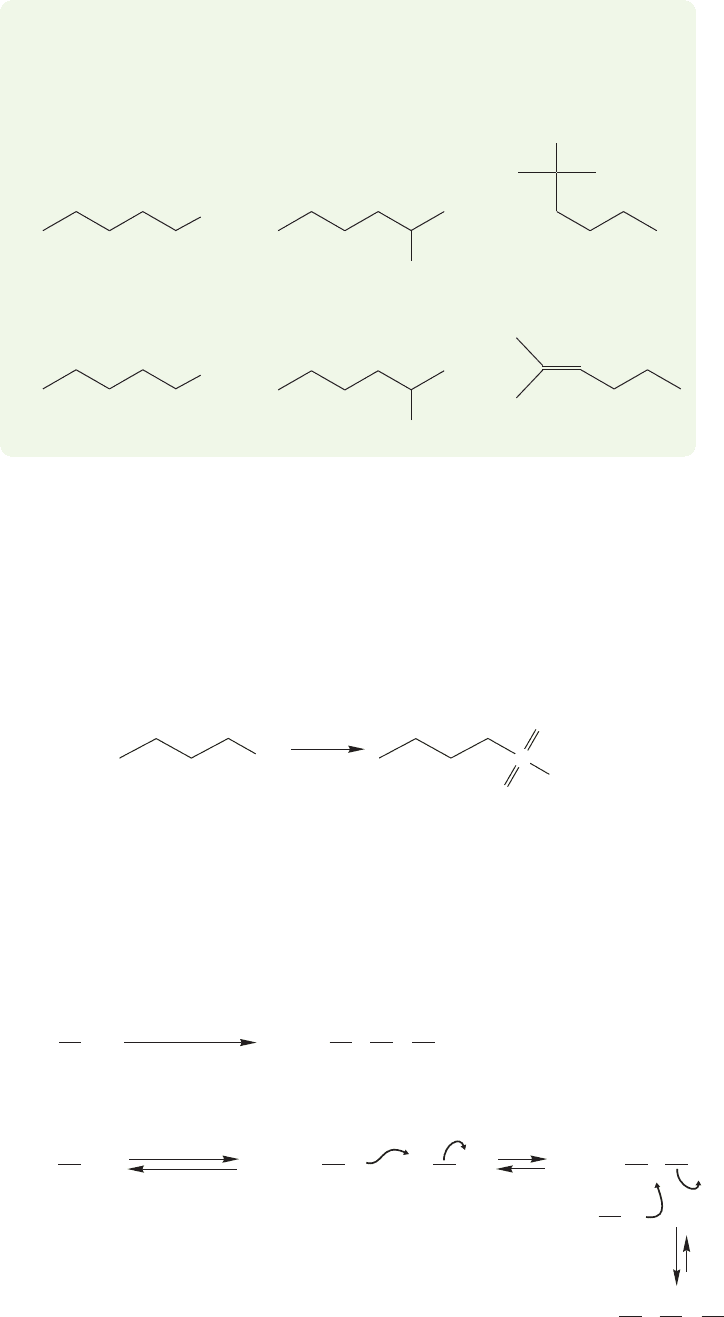
Like alcohols, thiols (mercaptans) can be oxidized,but oxidation usually takes place
not at carbon, as with alcohols, but on sulfur to give sulfonic acids (Fig. 16.76).
HNO
3
SH
Butanethiol Butanesulfonic acid
(85%)
S
O
O
OH
FIGURE 16.76 Oxidation of thiols
(mercaptans) with nitric acid gives
sulfonic acids.
KHCO
3
, 25 ⬚C
KHCO
3
SH
CH
3
CH
2
(95%)
..
..
SH
..
..
..
CH
3
CH
2
S
Br
2
CH
2
Cl
2
CH
3
CH
2
S
CH
2
CH
3
..
..
..
..
S
CH
3
CH
2
..
..
S
CH
3
CH
2
..
..
S
..
..
BrCH
3
CH
2
–
–
–
..
..
..
Br
..
..
..
Br
..
..
..
..
+
..
..
Br
..
..
–
..
..
Br
..
..
+
..
SCH
3
CH
2
S
CH
2
CH
3
..
.. ..
FIGURE 16.77 Mild oxidation with bromine
(or iodine) in base gives disulfides.
Milder oxidation, using halogens (I
2
or Br
2
) in base, is a general source of disulfides.
This reaction probably involves a sequence of displacement reactions (Fig. 16.77).
810 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
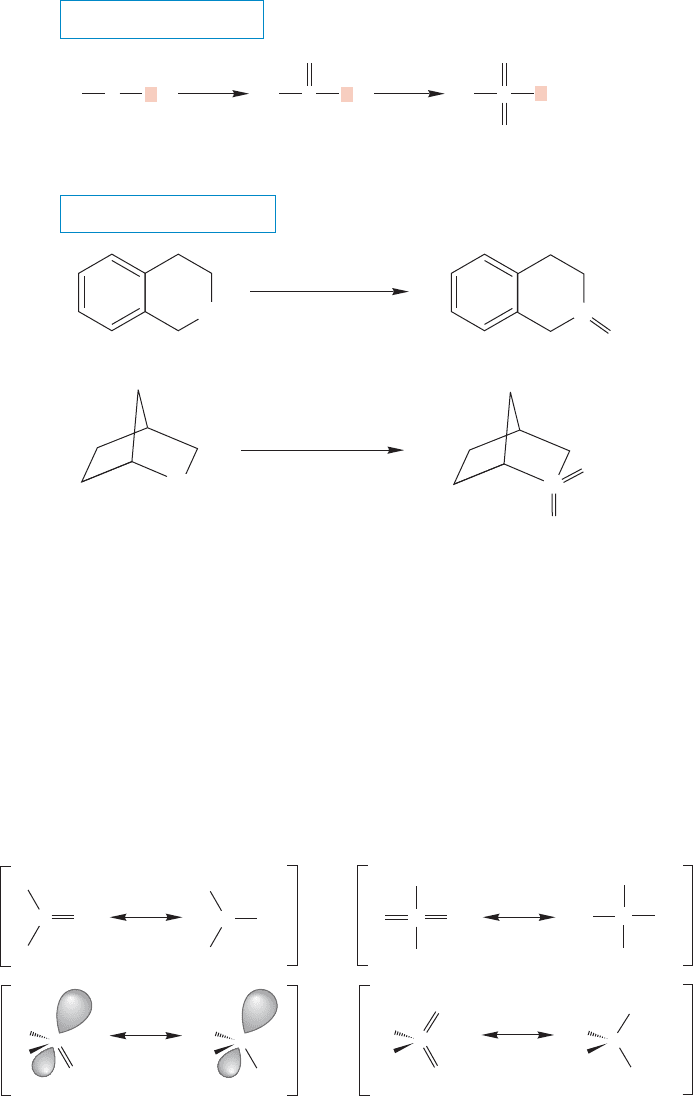
Unlike ethers, sulfides (thioethers) can easily be oxidized to sulfoxides, usually
with hydrogen peroxide,H
2
O
2
. Sulfoxides have the general formula R
2
SO.Further
or more vigorous oxidation gives a sulfone, a compound of the formula R
2
SO
2
(Fig. 16.78).
H
2
O
2
S
O
(76%)
(90%)
SR
Sulfoxide
Sulfide
Sulfone
30% H
2
O
2
CH
3
OH, NaOH
CH
3
COOH
..
..
SR
..
..
..
..
..
O
SR
..
..
O
..
..
H
2
O
2
O
..
..
S
CH
3
COOOH
⬚
50 C
O
..
..
O
..
..
..
S
S
..
..
R R
R
THE GENERAL CASE
SPECIFIC EXAMPLES
FIGURE 16.78 Sulfides can be oxidized to sulfoxides with hydrogen
peroxide. Further oxidation gives sulfones.
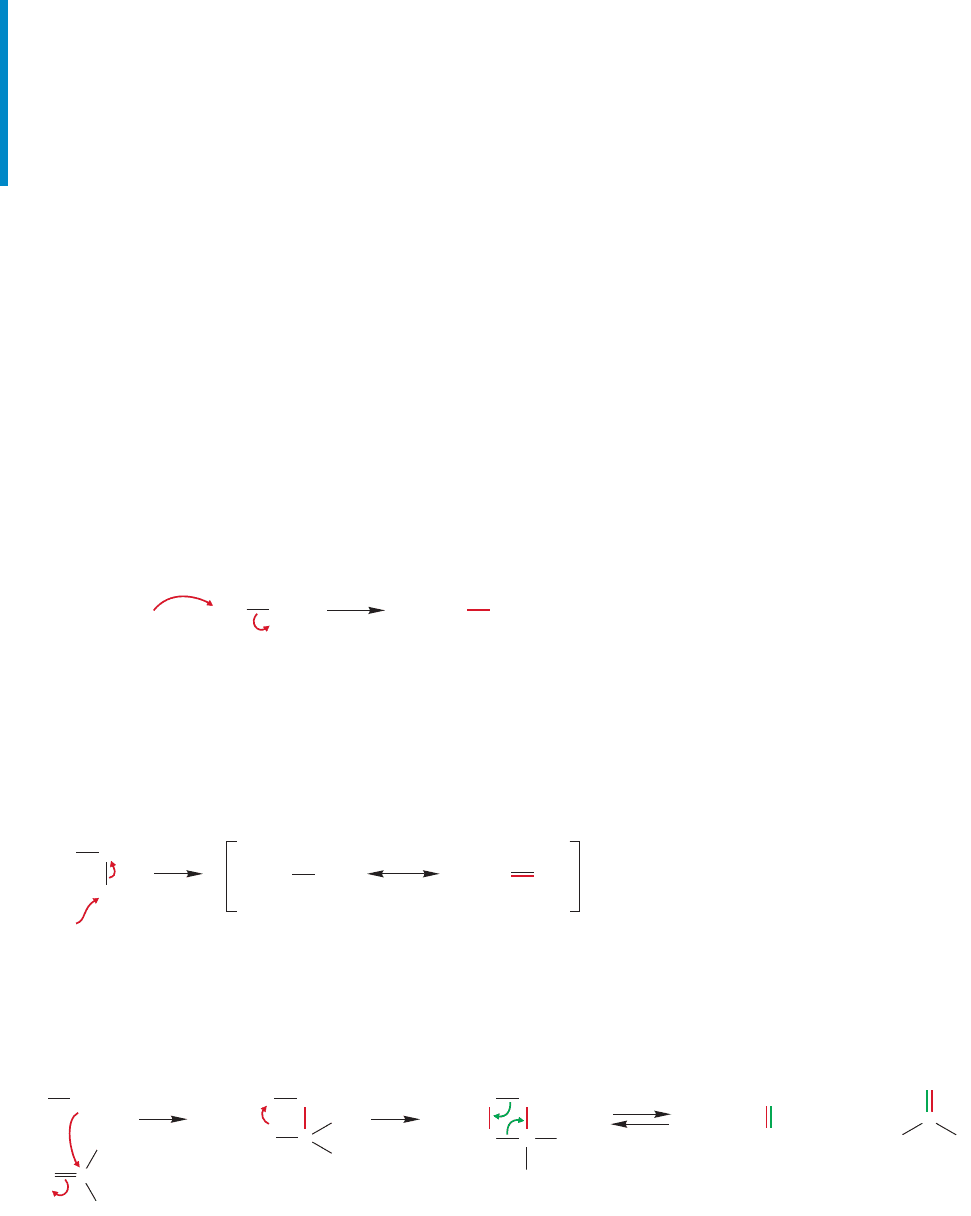
Sulfoxides and sulfones are more complicated structurally than they appear.
First, they are not planar, but approximately tetrahedral.Second, the uncharged res-
onance forms are not the major contributors to the structures. Better representa-
tions involve charge-separated resonance forms in which the sulfur octet is not
expanded (Fig. 16.79).
S
R
R
O
Sulfoxides
S
R
R
..
O
..
..
S
R
R
R
R
..
..
O
..
..
+
+
–
– –
–
S
..
..
O
..
..
O
..
..
R
R
S
O
..
..
O
..
..
..
..
..
..
S
R
R
S
R
R
O
–
..
..
S
R
R
O
..
..
2+
2+
..
–
O
..
..
..
..
O
..
..
O
..
..
Sulfones
FIGURE 16.79 A resonance description of sulfoxides and sulfones.
16.17 The Wittig Reaction 811
Summary
Thiol and sulfide chemistry resembles alcohol and ether chemistry.There are dif-
ferences,but many of the differences are quantitative, not fundamental. For exam-
ple, we have learned that mercaptides (RS
) are much better nucleophiles than
alkoxides (RO
), but even an alkoxide can undergo the S
N
2 reaction given the
right conditions and reagents.There are areas in which substantial differences do
appear; for example, sulfur is far more prone to oxidation than oxygen.
16.17 The Wittig Reaction
As we have seen, all manner of nucleophiles add to carbon–oxygen double bonds,
often leading to useful compounds such as alcohols. Now we will learn about a syn-
thetically useful carbonyl addition reaction that produces certain kinds of alkenes.
This reaction was discovered in the laboratories of Georg Wittig (1897–1987) and
is called the Wittig reaction.Wittig shared the 1979 Nobel prize in chemistry large-
ly for his work on this reaction.The process begins with the reaction between phos-
phines, (R
3
P), and alkyl halides to give phosphonium halides (Fig. 16.80). Although
we have not encountered reactions of phosphorus yet, it sits right below nitrogen in
the periodic table and, like nitrogen, is a good nucleophile. Formation of the phos-
phonium halide takes place through an S
N
2 displacement.
A phosphonium ion
CH
3
CH
3
+
(Ph)
3
P
..
I
..
..
..
S
N
2
(Ph)
3
P
–
I
..
..
..
..
Triphenyl-
phosphine
+
FIGURE 16.80 Displacement
of iodide by the nucleophilic
phosphorus atom of
triphenylphosphine leads to
phosphonium ions.
The phosphonium ion contains acidic hydrogens that can be removed by
strong bases such as alkyllithium reagents. The product is an ylide (pronounced
ill-id), a compound containing opposite charges on adjacent atoms (Fig. 16.81).
+
–
+
CH
2
(Ph)
3
P
+
H
–
..
Bu
Li
+
An ylide Butane
CH
2
(Ph)
3
P
..
CH
2
(Ph)
3
P
BuH
FIGURE 16.81 Protons adjacent to
the positively charged phosphorus
atom can be removed in strong base
to give ylides.
+
O
C
..
..
..
CH
2
–
CH
2
R
R
(Ph)
3
P
..
CH
2
(Ph)
3
P
O
C
..
R
R
..
–
..
CH
2
P
O
C
..
R
R
(Ph)
3
An oxaphosphetane
O
..
..
(Ph)
3
P
Triphenylphosphine
oxide
R
R
Product
++
C
alkene
FIGURE 16.82 Ylides are nucleophiles and will add to carbonyl compounds to give intermediates that can close
to oxaphosphetanes.These four-membered ring compounds can open to give triphenylphosphine oxide and the
product alkenes.
The carbon of the ylide is a nucleophile, and like other nucleophiles, adds to
carbon–oxygen double bonds (Fig. 16.82). Intramolecular closure of the intermediate
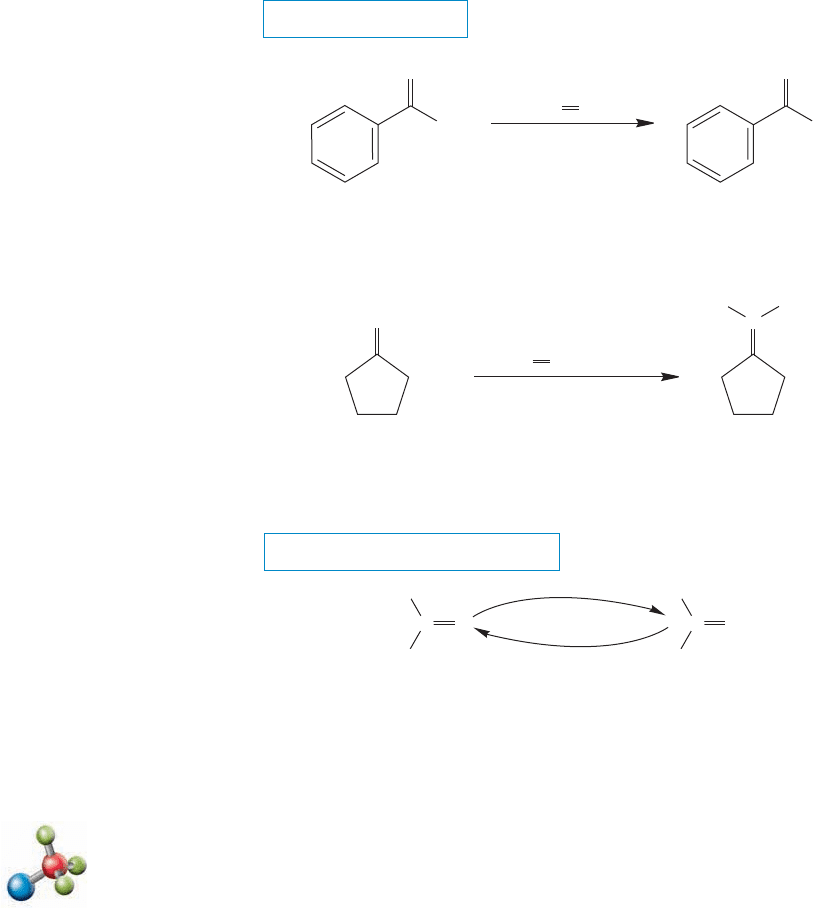
812 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
leads to a four-membered ring,an oxaphosphetane. Oxaphosphetanes are strained
and unstable relative to their quite stable constituent parts, phosphine oxides and
alkenes. Therefore, it is easy for these intermediates to fragment.
The Wittig synthesis does contain some unfamiliar intermediates. You have
not seen either an ylide or an oxaphosphetane before,for example.Yet the key reac-
tions, the S
N
2 displacement to form the phosphonium halide (Fig. 16.80) and the
addition to the carbonyl group (Fig. 16.82), are simple variations on basic process-
es. In a practical sense, it is worth working through the new parts of the Wittig
reaction because it is so useful synthetically. Note that the Wittig reaction is, in a
formal sense, the reverse of ozonolysis (p. 436). Figure 16.83 shows some specific
examples.
O
C
R
R
CH
2
C
R
R
Wittig
ozonolysis
GENERAL INTERCONVERSIONS
(43%)
10 h, 170 ⬚C
CHCOOC
2
H
5
(Ph)
3
P
O C
H
COOC
2
H
5
12 h, 65 ⬚C
ether
OCH
2
CH
2
(Ph)
3
P
(67%)
H H
SPECIFIC EXAMPLES
FIGURE 16.83 Some typical synthetic uses of the Wittig reaction. It converts
an aldehyde or ketone into an alkene.
The Wittig reaction is very useful. How else could we convert cyclohexanone
into pure methylenecyclohexane? We can use the reactions of this chapter to devise
a different synthesis. Some thought might lead to a sequence in which cyclohexa-
none reacts with methyllithium to give, after hydrolysis, a tertiary alcohol. An acid-
catalyzed elimination reaction would give some of the desired product, but there is
no easy way to avoid the predominant formation of the undesired isomer, 1-methylcy-
Wittig reaction
16.18 Special Topic: Biological Oxidation 813
..
..
..
..
HO
CH
3
O
2. H
2
O
1. CH
3
Li
H
2
O
H
3
O
+
+
CH
3
MinorMajor
CH
2
+
(a)
O
NaH
DMSO
(86%)
CH
2
(b)
Ph
3
P CH
3
FIGURE 16.84 (a) The dehydration approach fails to give very much of the desired
product. (b) The Wittig reaction is the preferred alternative.
(a) (b)
16.18 Special Topic: Biological Oxidation
We have seen several examples of redox reactions in the last few sections. Related
processes are of vital importance in biological systems. We humans, for example,
derive much of our energy from the oxidation of ingested sugars and fats.However,
we do not use chromium reagents or nitric acid as oxidizing agents to accomplish
these reactions, for these reagents are far too unselective and harsh. They would
surely destroy most of our constituent molecules; a quite uncomfortable process one
imagines.Instead, a series of highly selective oxidizing biomolecules has evolved, each
dedicated to a particular purpose. One of these, nicotinamide adenine dinucleotide
(NAD
),
3
is the subject of this section. Recall that we first looked at this molecule
in Chapter 14 (p. 679).
3
A glance at the structure of this molecule, and some brief thoughts on how complicated its systematic name
must be, quickly reveal the obvious reasons why biochemists are addicted to acronyms.
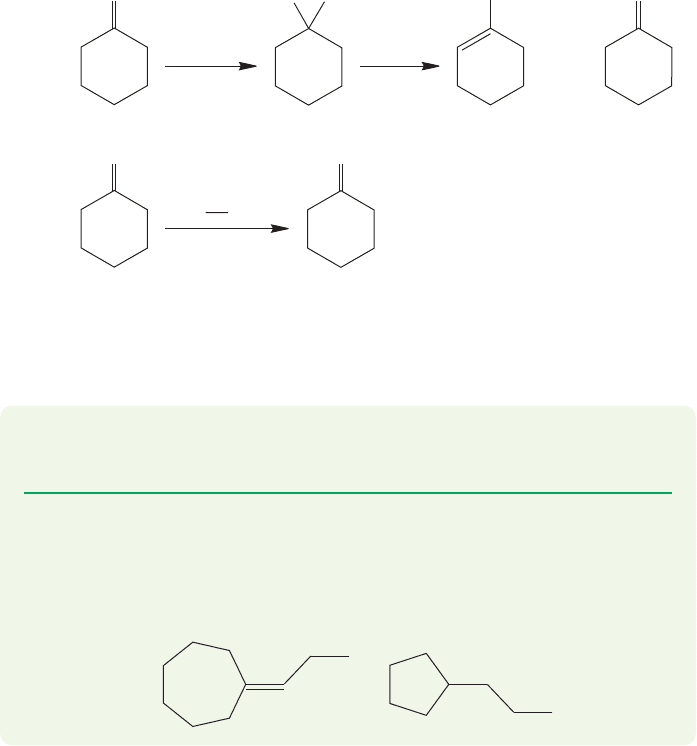
PROBLEM 16.28 Explain why the acid-catalyzed dehydration reaction of Figure
16.84a will give mostly the undesired 1-methylcyclohexene.
PROBLEM 16.29 Starting with cycloheptanone, cyclopentanone, triphenylphos-
phine, propyl iodide, butyllithium, and inorganic reagents of your choice, devise
synthesis of the following molecules:
clohexene (Fig. 16.84). The Wittig reaction solves this synthetic problem and is an
important constituent of any synthetic chemist’s bag of tricks.
814 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
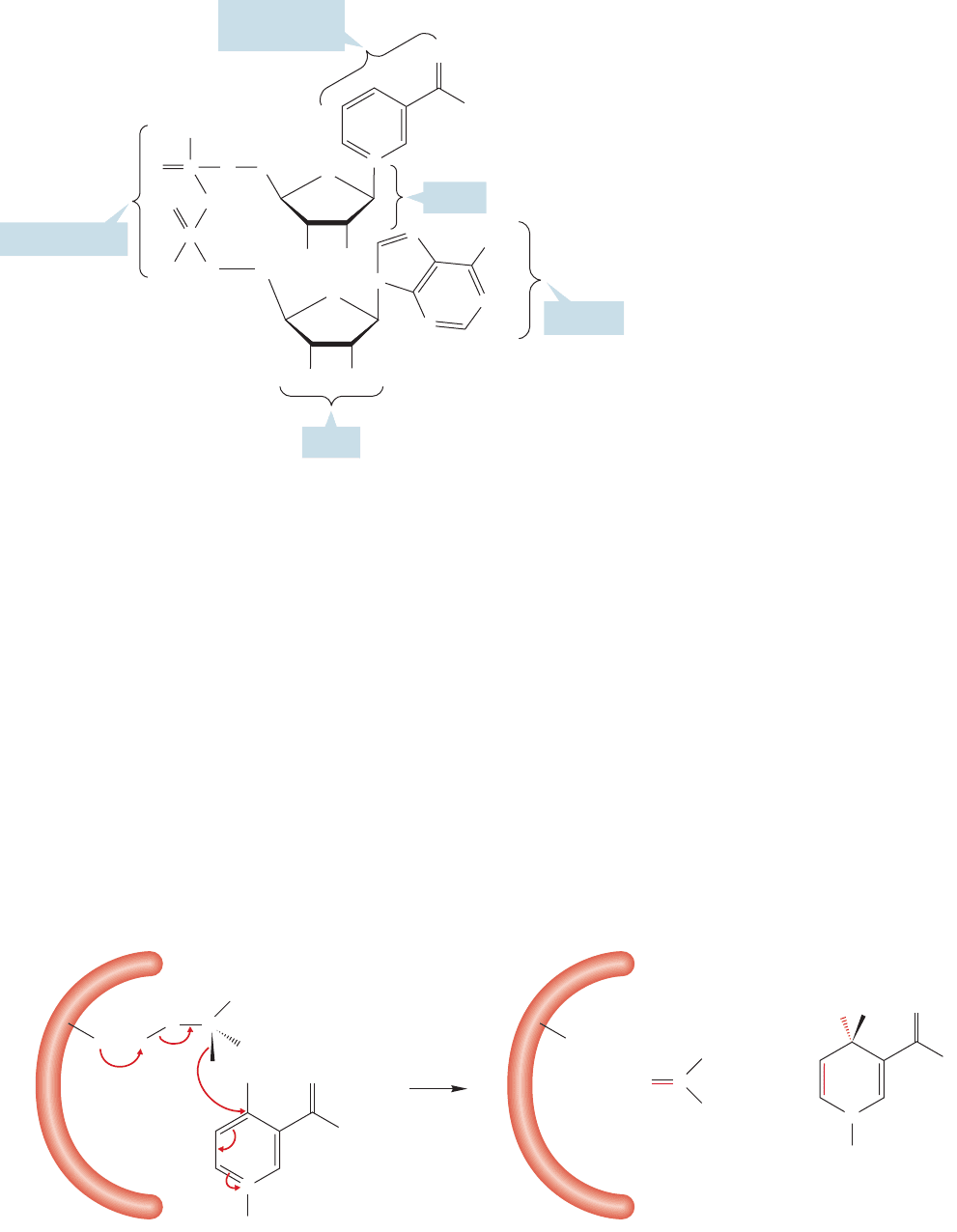
Nicotinamide adenine dinucleotide
is a pyridinium ion, and its full structure
is shown in Figure 16.85. The business
end of the molecule,the pyridinium ion,
is connected through a sugar (ribose)
and a pyrophosphate linkage to a second
ribose which is, in turn, attached to the
base adenine. The molecule is a coen-
zyme, a molecule needed by an enzyme
to carry out a reaction. In this case,
the enzyme is alcohol dehydrogenase.
The enzyme binds the alcohol, which is
the molecule to be oxidized, and NAD
,
which will be reduced.Ethanol is a com-
mon alcohol in our diet (it is in bread,
fruit, flavoring, and often in our drinks)
and it is on this alcohol that much of the
research on the mechanism of NAD
has been performed.
In this reaction, the NAD
behaves
as a Lewis acid, and accepts a hydride
ion. Ethanol is a Lewis base in this set-
ting,because it donates the H
.The enzyme serves to bring the NAD
and the ethyl
alcohol together, but this bringing together is of immense importance to the reac-
tion, as it allows the redox process to take place without the requirements that mol-
ecules in solution have for finding each other and orienting properly for reaction.
The NAD
is a strong acid by virtue of its positively charged quaternary nitro-
gen.It can be reduced by transfer of a hydrogen with its pair of electrons (a hydride reduc-
tion), from ethyl alcohol (Fig. 16.86). The reaction is completed by deprotonation to
give the product acetaldehyde and removal of the products from the enzyme. This
removal is easy because the enzyme has evolved to bind ethyl alcohol and NAD
, and
not acetaldehyde and NADH, the reduced form of NAD
. It is the acetaldehyde that
causes most of the health problems associated with significant ethanol consumption.
We will see NADH again in Chapter 23 as a biological hydride source.
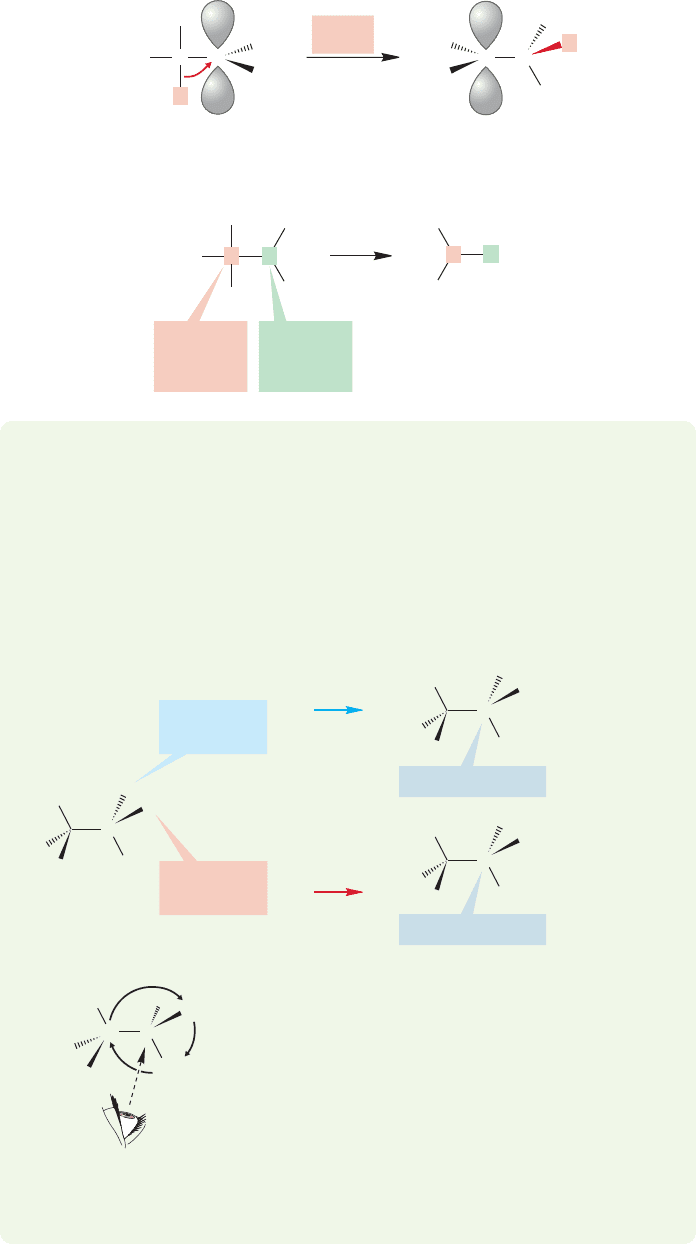
This hydride reduction may seem strange, but it is not. There is a close relative
in the intramolecular hydride shifts in carbocation chemistry. These, too, are redox
..
..
..
O
C
H
H
HB
H
..
..
O
NH
2
N
R
+
CH
3
Ethanol
NAD
+
+
BH
..
..
O
C
H
CH
3
Acetaldehyde
..
..
HH
..
..
O
NH
2
N
R
NADH
+
FIGURE 16.86 The NAD
is reduced by transfer of hydride (H
) from ethanol.
O
O
–
P
O
O
NH
2
NH
2
N
+
O
OH
HO
OH
HO
CH
2
O
N
N
O
CH
2
O
P
O
–
O
N
Sugar
The pyridinium
ion
Adenine
Sugar
N
Pyrophosphate
FIGURE 16.85 Nicotinamide adenine dinucleotide, NAD
.
16.18 Special Topic: Biological Oxidation 815
CH
3
+
+
C
H
3
C
H
C
CH
3
H
H
hydride
shift
C
Oxidized
in the
reaction
+
+
C
H
3
C
H
CH
3
H
3
C
C
H
C
H
3
C
H
CH
3
H
3
C
Secondary
carbocation
Tertiary
carbocation
Reduced
in the
reaction
C
CH
2
CH
3
H
3
C
H
3
C
..
..
..
..
FIGURE 16.87 Hydride shifts in
carbocations are also redox reactions.
WORKED PROBLEM 16.30 (a) Each methylene hydrogen of ethanol can be replaced
by deuterium to give a pair of chiral deuterioethanols. Show this process and indi-
cate which of the new compounds is (R) and which is (S). (b) Oxidation of (R)-
1-deuterioethanol produces deuterio-NADH (NADD) and acetaldehyde.Predict the
products of the oxidation of (S)–1–deuterioethanol with NAD
. Explain carefully.
ANSWER (a) Replacement of one methylene H with D gives the (S) enantiomer;
replacement of the other gives the (R) enantiomer.The hydrogens are enantiotopic.
H
H
H
H
This structure shows the priorities for the (
R ) compound;
the 1 ... 2 ... 3 arrow is clockwise
C
H
OH
Replace this
hydrogen
Replace this
hydrogen
H
H
D
H
C
H
OH
H
D
H
H
C
H
OH
This carbon is (S)
This carbon is (R)
H
D
3
H
H
C
H
1
OH
C
2
(b) The product will be 1-deuterioacetaldehyde. The binding site of the enzyme
is chiral and therefore the enantiotopic hydrogens are distinguishable.
reactions.The originally positive carbon is reduced and the carbon from which the
hydride moves is oxidized (Fig. 16.87).
816 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
16.19 Summary
New Concepts
The centerpiece of this chapter and its unifying theme is the
addition reaction in which nucleophiles of all kinds add to car-
bonyl groups. This process can be either acid- or base-catalyzed,
and starts with the overlap of a filled orbital on the nucleophilic
Lewis base with the empty π* orbital of the carbonyl to give an
alkoxide in which the highly electronegative oxygen atom bears
the negative charge (Fig. 16.22). The π system of the carbonyl
group is constructed through combinations of carbon and
oxygen 2p orbitals.This process leads to the π orbitals of
Figure 16.4.
Many addition reactions are equilibria, and in these cases
the position of the equilibrium depends on the relative stabili-
ties of the starting materials and products. These stabilities
depend, as always, on a variety of factors including electronic
structure, substitution pattern, and steric effects.
The close relationship between alcohols and carbonyls has
become apparent in this chapter. Oxidation of alcohols produces
the carbonyl group and reduction of the carbonyl, by hydrides
or organometallic reagents, produces the alcohol. Protecting
groups for alcohols, aldehydes, and ketones were introduced in
Section 16.10.
The concept of retrosynthetic analysis appears in this chap-
ter. Retrosynthetic analysis simply means that it is best to
approach problems of synthesis by searching not for the ulti-
mate starting material, but rather to undertake the much easier
task of finding the immediate precursor of the product. This
process can be repeated until an appropriate level of simplicity
is reached (Fig. 16.88).
Key Terms
acetal (p. 786)
benzaldehyde (p. 768)
carbinolamine (p. 790)
coenzyme (p. 814)
cyanohydrin (p. 781)
dial (p. 767)
dione (p. 768)
enamine (p. 795)
gem-diol (p. 776)
hemiacetal (p. 783)
hydrate (p. 773)
imine (p. 792)
iminium ion (p. 795)
oxaphosphetane (p. 812)
protecting group (p. 788)
retrosynthetic analysis (p. 807)
Schiff base (p. 792)
silyl ether (p. 789)
sulfone (p. 810)
sulfoxide (p. 810)
tetrahydropyranyl (THP) ether (p. 789)
Wittig reaction (p. 811)
ylide (p. 811)
Reactions, Mechanisms, and Tools
As mentioned in the New Concepts section, there is a single,
central reaction in this chapter, the addition of a nucleophile to
the Lewis acid carbonyl group.The details of the mechanism
vary, depending on whether the reaction is acid-catalyzed, base-
catalyzed, reversible, or irreversible. Here are some general
examples.
A simple case is the acid- or base-catalyzed reversible addi-
tion reaction (Fig. 16.35). Examples are hydration or cyanohy-
drin formation.
A slightly more complicated reaction involves an addition
followed by loss of water. An example is the reaction of primary
amines with carbonyl groups to give substituted imines
(Fig. 16.51).
If there is no proton that can be removed after loss of water,
a second addition reaction can occur, as in acetal formation
(Fig. 16.43).
There are also irreversible additions, as in the reactions of
organometallic reagents or metal hydrides with carbonyl com-
pounds. Protonation of the initial products, alkoxides, gives
alcohols (Fig. 16.59).
This chapter also introduces the oxidation reactions of
alcohols. These reactions involve addition reactions in their
first steps. For example, in the oxidation of primary alcohols
by CrO
3
, the first step is addition of the alcohol to the
chromium–oxygen double bond (Figs. 16.67 and 16.68).
The oxidation is completed by an elimination in which the
new carbon–oxygen double bond is constructed.
Target
molecule
Last
precursor
molecule
Next
precursor
molecule
Simple
starting
material
FIGURE 16.88 Retrosynthetic analysis.
16.19 Summary 817
Syntheses
This chapter contains lots of new syntheses.
1. Acetals
2. Acids
3. Alcohols
The alkoxide is an intermediate;
LiAlH
4
can also be used
R
H
O
C
1. NaBH
4
2. H
2
O
H
H
O
C
1. RMgX or RLi
2. H
2
O
The alkoxide is an intermediate
Primary alcohols
R
OH
H
C
H
R
OH
H
C
H
Other oxidizing agents such as KMnO
4
work also.
The aldehyde and its hydrate are intermediates
R
H
O
C
R
HO
O
C
Na
2
CrO
4
H
3
O
+
H
3
O
+
R
OH
H
C
R
HO
O
C
Na
2
CrO
4
H
Acetals can be used as protecting groups for
aldehydes and ketones; treatment with H
2
O/H
3
O
+
regenerates the aldehyde. The hemiacetal
is an intermediate
R
H
O
C
ROH
2
ROH
+
OR
R
C
RO
H
O
C
ROH
2
ROH
+
OR
R
C
RO
R
R
R
4. Aldehydes
5. Alkenes
The Wittig reaction
R
R
O
C
R
R
C
(Ph)
3
P CR
2
CR
2
Hydrolysis of an acetal
R
H
O
C
H
3
O
+
H
2
O
HIO
4
H
2
O
OR
R
C
RO
H
Oxidation of a primary alcohol; water must be absent
Periodate cleavage of a vicinal diol; reaction
involves a cyclic intermediate
R
H
O
C
R
H
O
C2
CrO
3
pyridine
R
OH
OHHO
RR
H
C
H
The alkoxide is an intermediate;
the R groups can be the same or different
R
R
O
C
1. NaBH
4
2. H
2
O
R
H
O
C
2. H
2
O
The alkoxide is an intermediate; the R groups can be
the same or different, depending on the structure of
the ketone; LiAlH
4
can also be used
Secondary alcohols
1. RMgX or RLi
R
OH
H
C
R
R
OH
H
C
R
R
R
O
C
2. H
2
O
Tertiary alcohols
1. RMgX or RLi
R
OH
R
C
R
The product can be R
3
COH, R
2
RCOH, or
RRRCOH, depending on the structures
of the starting ketone and organometallic reagent
818 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
6. Alkylithium Reagents
7. Bisulfite Addition Products
8. Cyanohydrins
9. Disulfides
10. Enamines
11. Grignard Reagents
12. Hemiacetals
Rarely isolable; exceptions are cyclic hemiacetals,
especially sugars; the reaction usually goes
further to give the full acetal
R
H
O
C
OH
R
C
H
ROH
2
, ROH
RO
+
R
R
O
C
OH
R
C
R
ROH
2
, ROH
RO
+
Mg
ether
RRMgX
X
The structure of the organometallic reagent is complicated,
as RMgX is in equilibrium with other molecules; the ether
solvent is also critical to the success of the reaction
At least one
α-hydrogen must be available;
the amine must be secondary
R
O
R
R
2
NH
NR
2
C
C
α-Position
R
R
I
2
base
RSH RSSR
KCN
H
2
O
Cyanohydrin formation is more favorable
for aldehydes than for ketones
R
H
O
C
OH
C
H
R
CN
Na
+
HSO
3
–
H
2
O
Also works with some ketones
R
H
O
C
OH
C
H
R
SO
3
–
Na
+
Li
R RLi
X
X = Cl, Br, or I
RLi is a simplistic picture of the reagent, which is
not monomeric
13. Hydrates
14. Hydrocarbons
15. Imines
16. Ketones
O
C
OR
C
H
3
O
+
+
H
2
O
RO
The hydrolysis of an acetal
HO OH
RR
RR
O
RR
2
R
R
R
R
O
C
OH
C
H
3
O
Na
2
CrO
4
H
2
O
HIO
4
Many other oxidizing agents will oxidize secondary
alcohols to ketones
Periodate cleavage of a vicinal diol
involves a cyclic intermediate
R
H
R
R
R
R
H
O
C
R
H
C
RNH
2
NR
This reaction works for ketones as well;
many varieties of imine are known,
depending on the structure of R in the
amine, which must be primary
H
2
O
RMgXRH
R
2
Cu
–
Li
+
H
2
O
RLi RH
X
R
R
R
R X must be primary or secondary
Unstable; for ketones the starting material
is usually favored
More likely to be favored at equilibrium than
the hydrated ketones; not usually isolable
R
R
O
C
OH
R
C
R
+
H
2
O
HO
R
H
O
C
OH
R
C
H
H
3
O
+
H
3
O
H
2
O
HO
