Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

16.19 Summary 819
17. Lithium Organocuprates
18. Sulfones
RSR
+ H
2
O
2
excess
The sulfoxide is an intermediate
R
S
O
O
R
R
2
Cu
–
Li
+
+ LiX
RLi
+ CuX
X = I, Br, or Cl
19. Sulfonic Acids
20. Sulfoxides
RSH
+ H
2
O
2
S
O
RR
Further oxidation gives the sulfone
RSH
+ HNO
3
OH
S
O
O
R
Common Errors
By far the hardest thing about this chapter is the all-too-plentiful
detail. To get this material under control it is absolutely necessary
to be able to generalize. The easiest mistake to make is to
memorize the details and lose the broad picture. Although there
is one general principle in this chapter (nucleophiles add to
carbon–oxygen double bonds), it is easy to get lost in the myriad
protonation, addition, and deprotonation steps. The reversible
addition reactions of this chapter are the hardest to keep
straight. The following analysis is a model for the sorting process
necessary to focus on the fundamental similarities, while being
mindful of the small, but important differences introduced by
structural changes in the molecules involved in these reactions.
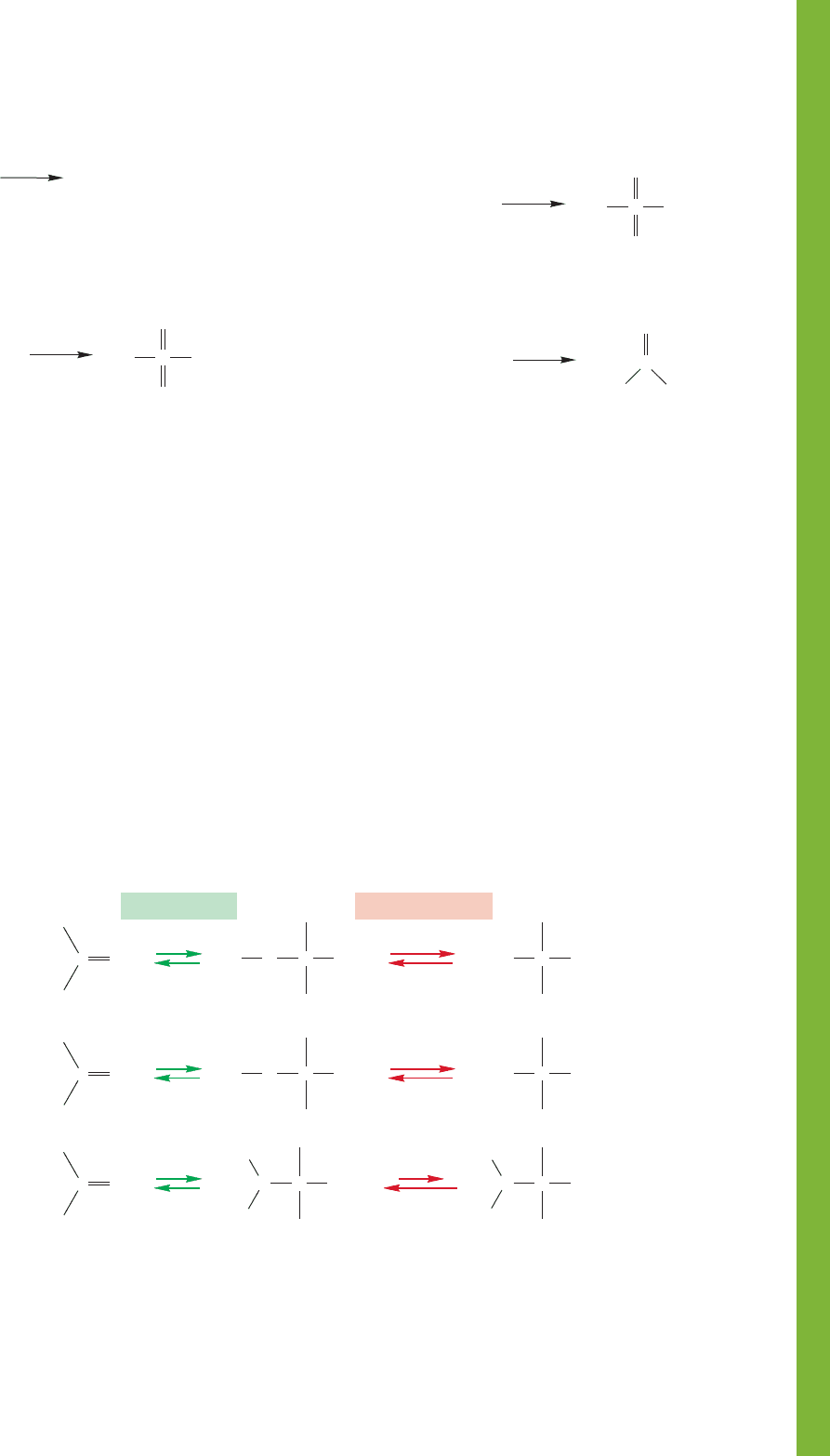
In base, hydroxide, alkoxides, and amide ions all add to
the carbonyl group to give alkoxides that can be protonated to
produce hydrates, hemiacetals, and carbinolamines. The three
reactions are very similar (Fig. 16.89).
R
H
..
..
..
..
H
2
O
+
O
O
..
..
O
..
..
..
HO
H
C
Hydrate
C
..
..
OH
..
..
HO
C
..
..
..
..
ROH
+
O
O
..
..
O
..
..
..
RO
R
C
Hemiacetal
C
..
..
OH
..
..
RO
C
..
..
..
..
..
..
..
RNH
2
+
O
O
..
..
..
RNH
C
Carbinolamine
C
..
..
OH
C
–
N
R
H
..
N
Addition steps Protonation steps
–
–
–
–
–
FIGURE 16.89 Base-catalyzed additions to carbonyl groups.
820 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
..
..
H
2
O
+
O
..
H
2
OR
C
Hydrate
..
..
OH
..
..
HO
C
..
ROH
OH
C
Hemiacetal
..
..
OH
..
..
RO
C
RNH
2
Carbinolamine
..
..
OH
C
R
H
..
N
Addition
steps
Protonation
steps
Deprotonation
steps
+
RNH
3
+
..
H
3
O
+
+
..
..
..
..
..
..
OH
C
+
..
..
OH
C
R
H
..
O
+
..
..
OH
C
R
H
N
+
..
..
OH
C
H
H
..
O
+
H
..
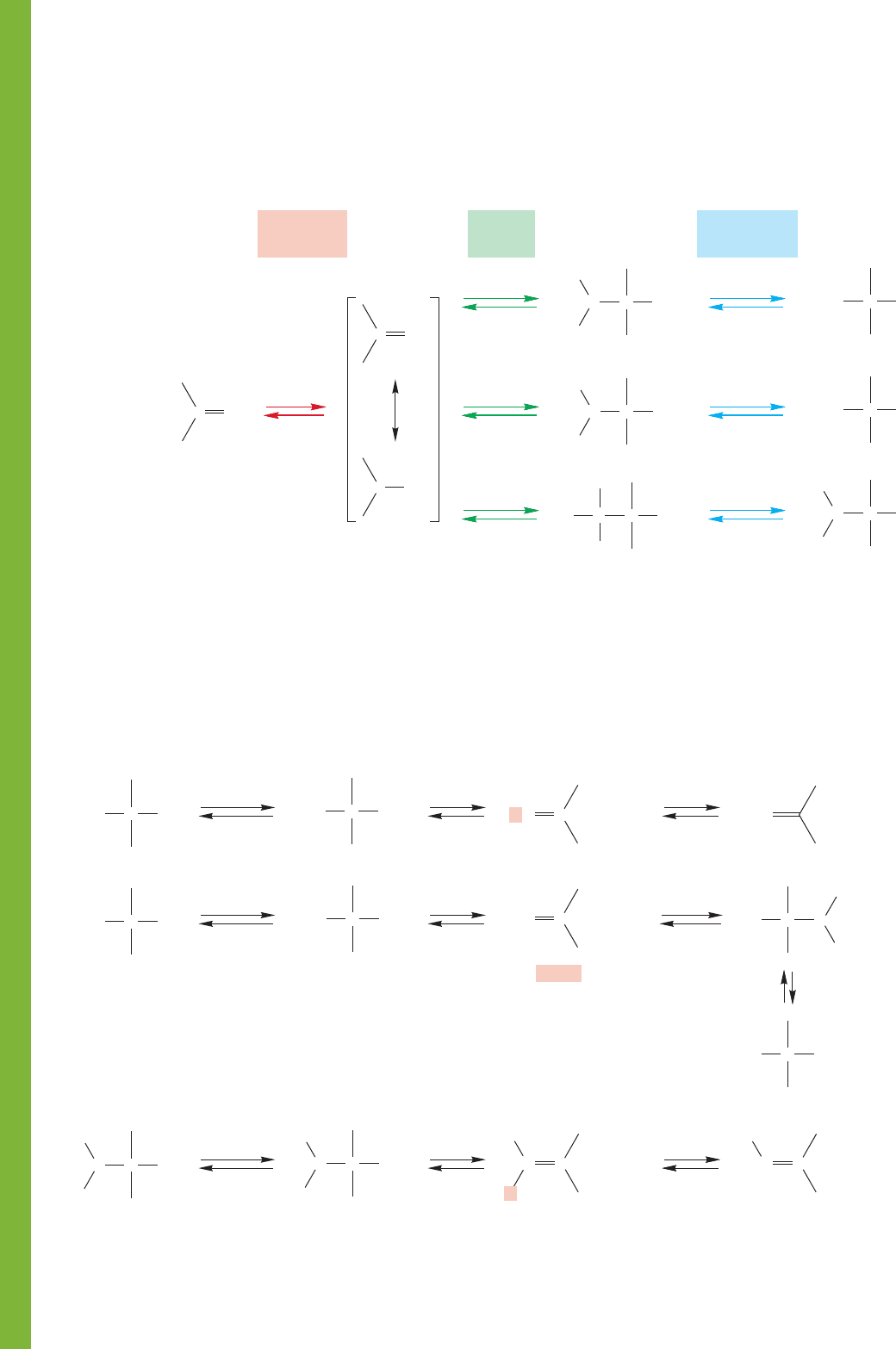
FIGURE 16.90 Acid-catalyzed additions to carbonyl groups.
Hydrate
..
..
OH
..
..
HO
C
Hemiacetal
..
..
OH
..
..
RO
C
Carbonyl
ROH
2
H
3
O
..
+
+
..
..
..
HO
..
HO
C
..
..
RO
C
..
OH
2
+
..
OH
2
+
..
..
..
RO
..
..
HOR
C
C
+
+
H
2
O
..
..
..
..
..
RO
C
+
+
+
H
2
O
..
..
..
O
+
H
3
O
O
+
ROH
2
..
(No proton
to lose!)
H
R
+
+
RNH
2
..
..
..
..
..
RO
C
OR
Full acetal
Carbinolamine Imine
..
..
OH
C
R
H
..
N
RNH
3
+
C
R
H
..
N
..
OH
2
+
..
N
C
R
+
+
H
2
O
..
N
C
R
+
+
RNH
3
..
H
FIGURE 16.91 Further reactions in acid of hydrates, carbinolamines, and hemiacetals.
In acid, similar reactions occur, and the three processes
begin with closely related sequences of protonation, addition,
and deprotonation (Fig. 16.90).
In the acid-catalyzed reactions, water can be lost from the
hydrate, hemiacetal, and carbinolamine to give new, resonance-
stabilized intermediates. In the hydrate, this results only in re-
formation of the original carbonyl group, but new compounds
can be produced in the other two cases. The carbinolamines
formed from primary amines follow a similar path that leads
ultimately to imines. Hemiacetals can lose water but do not
have a second proton that can be lost. Instead, they add a
second molecule of alcohol to give acetals (Fig. 16.91).
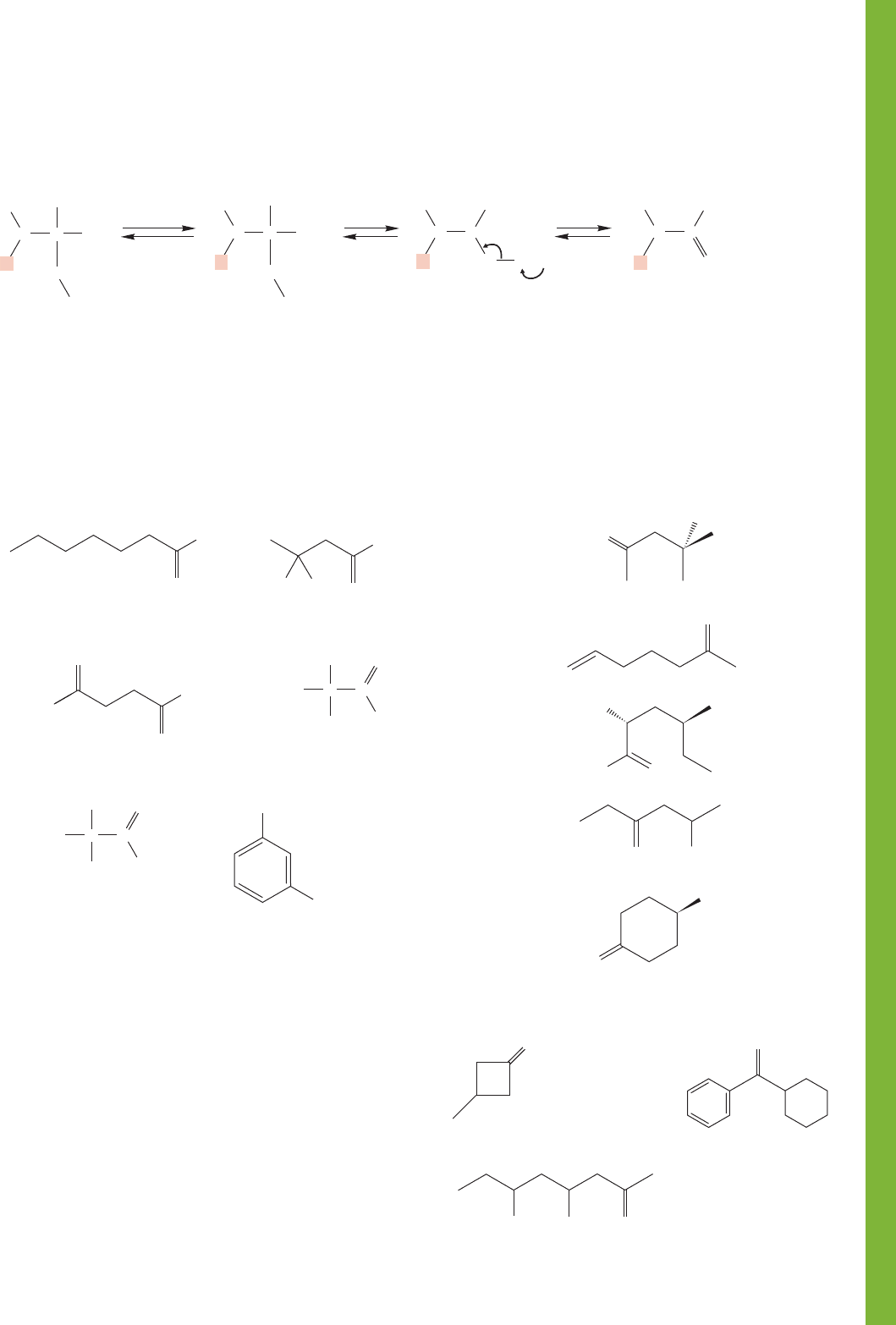
16.20 Additional Problems 821
Carbinolamine
Enamine
..
..
OH
C
H
2
C
R
R
..
N
C
R
R
R
R
.. ..
..
..
N
..
OH
2
+
N
C
RR
+
+
..
HOH
2
..
N
C
R
+
H
3
O
R
H
H
2
C
H
2
C
R
CH
2
H
R
+
..
H
3
O
FIGURE 16.92 Further reactions of carbinolamines formed from secondary amines.
16.20 Additional Problems
PROBLEM 16.34 Name the following compounds according to
the official naming system (IUPAC):
PROBLEM 16.31 Name the following molecules:
The carbinolamines formed from secondary amines and
carbonyl compounds also have no second proton to lose
and instead are deprotonated at carbon to give enamines
(Fig. 16.92).
(a)
(d)
H
O
O
(c)
(b)
H
H
O
Cl
Cl
O
H
H
3
C
C
H
3
C
H
3
C
C
O
H
(e)
(f)
ClCH
2
C
H
3
C
H
3
C
C
O
H
F
CHO
PROBLEM 16.32 Draw structures for the following
compounds:
(a) p-nitrobenzaldehyde
(b) 4-methylhexanal
(c) 2-methylpropiophenone (isobutyrophenone)
(d) cis-2-bromocyclopropanecarboxaldehyde
PROBLEM 16.33 Draw structures for the following
compounds:
(a) (R)-2-amino-3-heptanone
(b) 4-oxopentanal
(c) 3-hydroxycyclopentanone
(d) phenylacetone
(e) (E)-2-octenal
O
(a)
O
(b)
(c)
(d)
O
Br
(e)
Cl
H O
OCH
3
O
PROBLEM 16.35 Name the following compounds:
O
(a)
(c)
(b)
O
O
NH
2
NH
2
822 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
(IR,
1
H NMR)
(d)
H
3
C
C
CH
3
H
C
O
CH
3
H
3
C
C
O
CH
3
H
CH
2
H
C
(
13
C NMR)
(c)
O
O
(a)
(IR)
(IR)
(b)
O
O
O
O
PROBLEM 16.36 Draw structures for the following
compounds:
(a) methyl propyl ketone
(b) 2-pentanone
(c) 2,3-hexanedione
(d) butyrophenone
(e) 3-bromo-4-chloro-5-iodo-2-octanone
PROBLEM 16.37 Draw structures for the following
compounds:
(a) 3,5-di-tert-butyl-4-hydroxybenzaldehyde
(b) 2-acetylcyclopentanone
(c) 2,4-dichloro-4-nitrobenzophenone
(d) 6-methyl-2-pyridinecarboxaldehyde
(e) (E)-4-phenyl-3-buten-2-one
(f) aminoacetaldehyde dimethyl acetal
PROBLEM 16.38 Use the indicated spectroscopy to distinguish
the following pairs of isomers:
PROBLEM 16.39 Give the major organic products expected in
each of the reactions in the next column. Hint: Part (k) involves
an intramolecular reaction.
(a)
O
O
O
HO OH
benzene
cat. H
+
(c)
NH
(b)
PhNH
2
cat. H
+
cat. H
+
OH
(d)
C
O
CH
3
Ph
2. H
2
O/H
3
O
+
1. PhMgBr
(e)
C
O
Ph
Ph
2. H
2
O/H
3
O
+
1. CH
3
Li
(f)
C
O
CH
3
Ph
CH
3
OH
NaBH
4
(g)
C
O
Ph
Ph
2. H
2
O/H
3
O
+
1. LiAlH
4
(j)
pyridine
CrO
3
(i)
PhCH
2
OH
H
2
CrO
4
H
2
O
(k)
C
O
(CH
2
)
4
CH
2
I
Ph
2. BuLi
3.
Δ
1. Ph
3
P
(h)
H
2
SO
4
Na
2
Cr
2
O
7
OH
CH
3
Ph
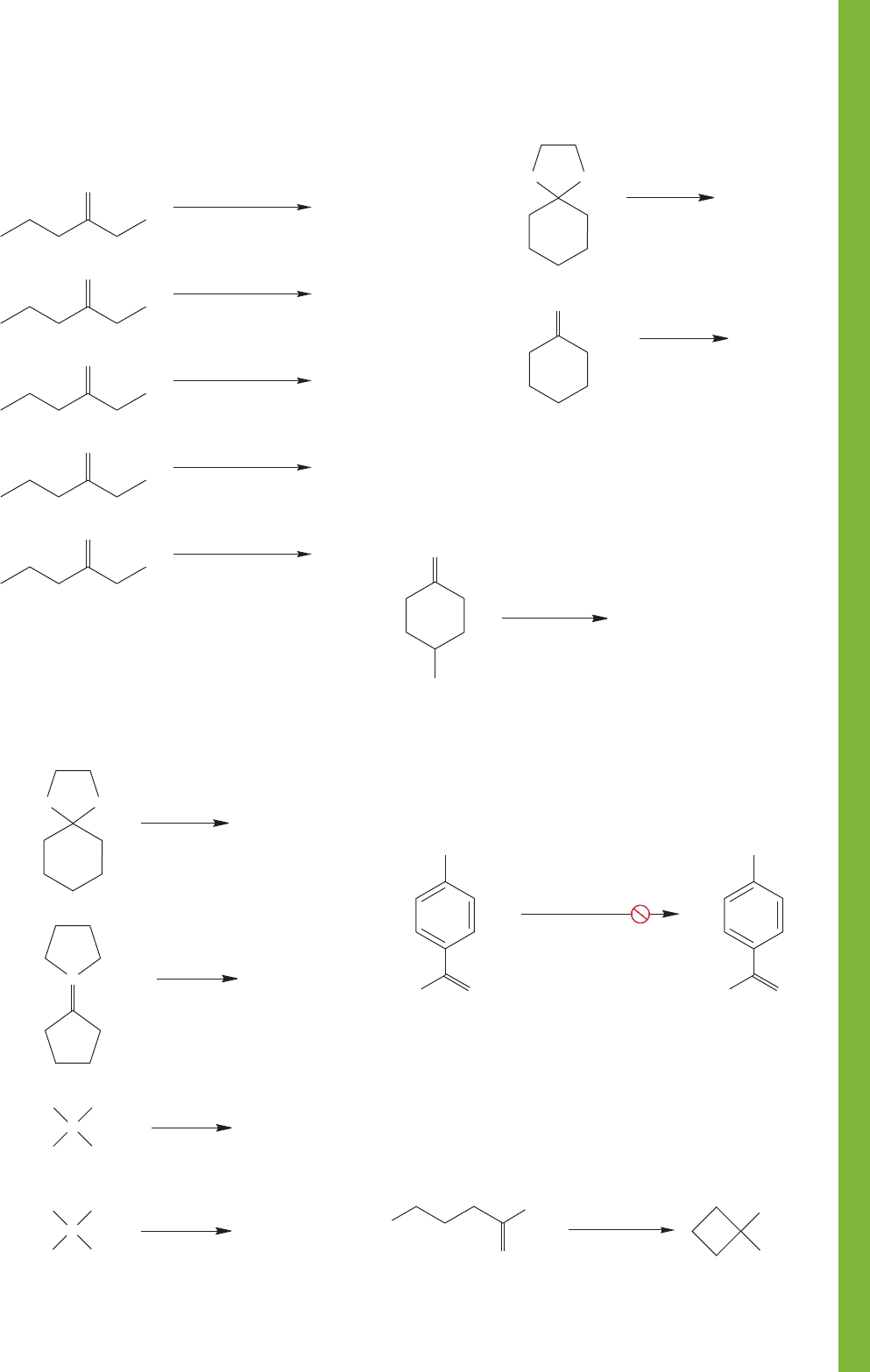
16.20 Additional Problems 823
PROBLEM 16.43
The reduction of 4-tert-butylcyclohexanone
with lithium aluminum hydride affords two isomeric products.
What are the structures of these two products? Which isomer
do you expect to predominate?
PROBLEM 16.45 Devise a scheme for avoiding the problem
you discovered in your answer to Problem 16.44. Hint:See
page 788.
PROBLEM 16.46 Write an arrow formalism for the following
reaction:
PROBLEM 16.44 As we saw on page 798, benzyl halides can
be reduced by metal hydrides. Why can compound A not be
reduced successfully with lithium aluminum hydride? What
reaction will complicate matters?
PROBLEM 16.41 Provide an argument not involving molecular
orbitals to explain why a carbon–oxygen double bond proto-
nates on O, not C.
PROBLEM 16.42 Write products and arrow formalism
mechanisms for the following simple changes (indicate NR if
no reaction is expected):
PROBLEM 16.40 Determine the product for each of the fol-
lowing reactions:
O
catalytic H
+
NH
2
OH
toluene, Δ
catalytic H
+
NH
2
CH
2
Ph
toluene, Δ
(b)
O
(a)
catalytic H
+
NH
2
NH
2
toluene, Δ
O
(c)
catalytic H
+
NH
2
NHPh
toluene, Δ
O
(d)
catalytic H
+
NH
2
NHCONH
2
toluene, Δ
O
(e)
(c)
CH
3
OH
2
CH
3
OH
(d)
(a)
H
2
O/H
3
O
+
(b)
H
2
O
OO
N
+
CH
3
C
H
3
C
H
3
CO
OH
+
KOH/H
2
O
CH
3
C
H
3
C
H
3
CO
OH
NH
2
OH
+
NH
3
OH
(e)
KOH/H
2
O
(f)
OO
O
Two isomeric products
1. LiAlH
4
2. H
2
O/H
3
O
+
C(CH
3
)
3
O
1. LiAlH
4
2. H
2
O
CH
2
Br
H
3
CO
CH
3
H
3
CO
A
O
CH
3
CH
3
OH
Br
1. Mg/THF
2. H
2
O/H
3
O
+
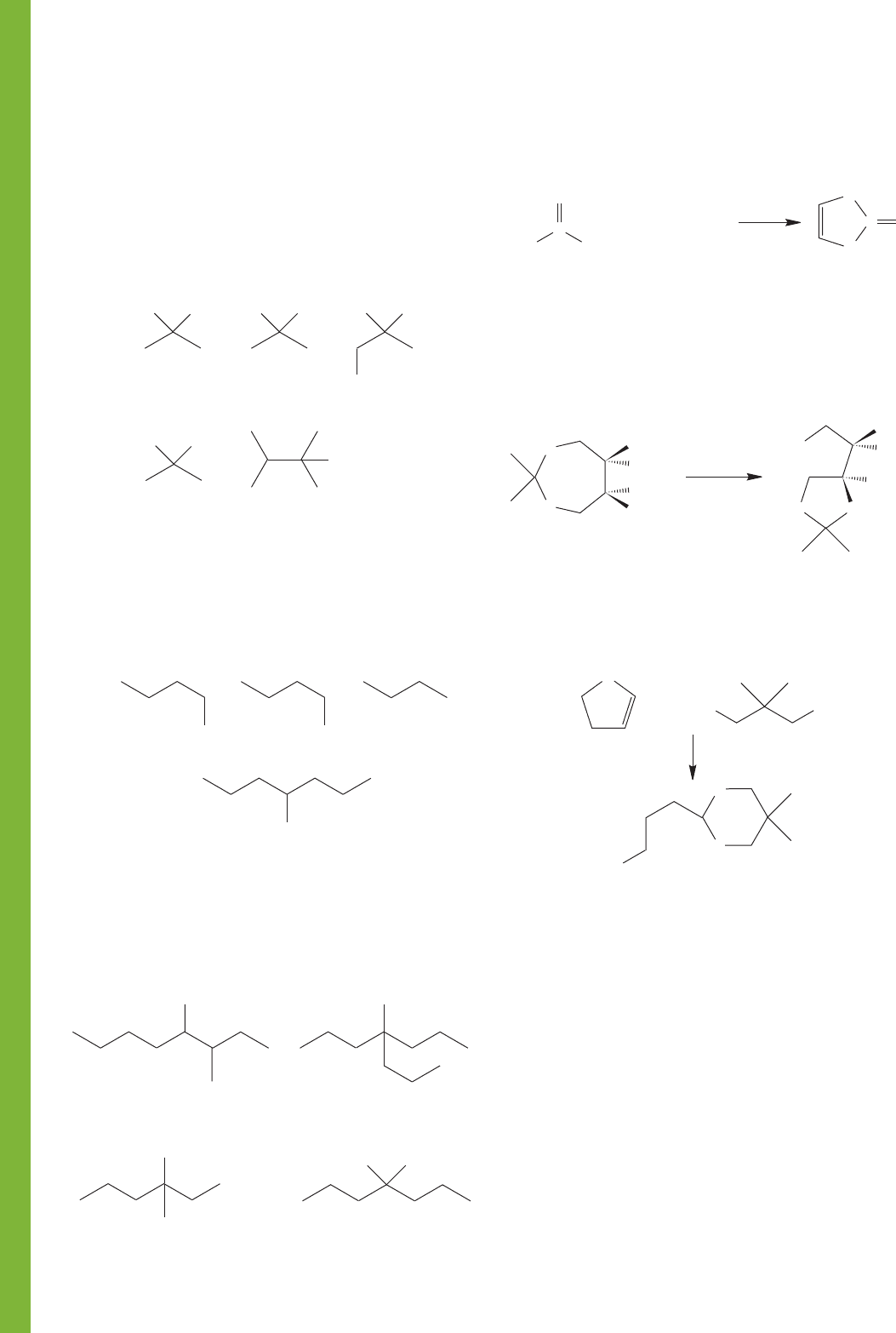
824 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
PROBLEM 16.53 In Section 16.10 we learned about protecting
groups for alcohols. One of the advantages for using a silyl ether
as a protecting group is that it can be selectively removed by treat-
ing with fluoride ion. The fluoride ion attacks the silicon atom
and breaks the silicon-oxygen bond. You know that the
bond is very strong. Why is it the bond that is broken
rather than one of the bonds in this reaction? If you are
familiar with the process of etching glass, you know this chemistry
is related. What acid do you suppose is used to etch glass?
PROBLEM 16.54 There are many reagents that have been
developed to oxidize alcohols to ketones or aldehydes. The
chromium reagents work very well, but other procedures are
preferred. Why do you suppose that other reagents are favored?
What are the hazards of using Cr(VI)?
Si
O
C
Si
O
O
Si
O
O
PROBLEM 16.52 Propose an arrow formalism mechanism for
the following reaction:
PROBLEM 16.51 Propose an arrow formalism mechanism for
the following reaction. What is the driving force for this reac-
tion? Why does it go from the seven-membered ring to the
five-membered ring?
PROBLEM 16.50 Propose an arrow formalism mechanism for
the following reaction:
PROBLEM 16.49 Starting from alcohols containing no more
than four carbon atoms, inorganic reagents of your choice, and
“special” organic reagents (such as ether and pyridine) provide
syntheses of the following molecules:
PROBLEM 16.48 Starting from propyl alcohol, organic reagents
containing no more than one carbon, and incidental organic
reagents such as bases and solvents, as well as inorganic materi-
als, devise syntheses of the following compounds. Some of the
necessary transformations will require conversions covered in the
earlier chapters. See, for example, figures on page 283.
PROBLEM 16.47 Starting from isopropyl alcohol as your
primary source of carbon, incidental organic reagents such as
bases and solvents, as well as inorganic reagents of your choice
(including labeled materials as needed), devise syntheses of
the following labeled compounds. It is not necessary to write
mechanisms.
D
H
(a)
D
D
H
(c)
D
D
(b)
D
OH
(d) (e)
D
(a)
Cl
(b)
CN
(d)
OH
(c)
(a)
OH
(b)
OH
(d)
OCH
3
H
3
CO
(c)
Br
C
O
NHCH
2
CH(OCH
2
CH
3
)
2
H
2
N
H
2
O
H
3
O
+
O
C
NH
NH
O
Δ
HCl
O
H
H
OH
CH
3
H
CH
3
H
OO
HO
OH
+
O
HCl
H
2
O
HO
HO
O
O

16.20 Additional Problems 825
PROBLEM 16.56
The reaction of acetone with methylamine
reaches equilibrium with a carbinolamine and, eventually, an
imine. There is another participant in the equilibrium called an
aminal. Its formula is C
5
H
14
N
2
. Provide a structure and a
mechanism.
PROBLEM 16.57 Treatment of aromatic compound A as shown
below leads to two products of the same formula, C
9
H
10
, B and
C. The
1
H NMR spectra of B and C are very similar except that
the coupling constant between two hydrogens at δ 6.3 is 8 Hz in
B and 14 Hz in C. Treatment of both B and C with ozone fol-
lowed by oxidative workup leads to benzoic acid and acetic acid.
Provide structures for B and C and explain your reasoning.
PROBLEM 16.61 Predict the product for the following reaction:
PROBLEM 16.60 Remember that most nucleophiles add
to carbonyl groups. Given that notion, and your answer to
Problem 16.59, provide a mechanism for the following
reaction:
PROBLEM 16.59 Hydrogens adjacent to carbonyl groups,
, are relatively acidic (pK
a
20). That is, they are rela-
tively easily removed by a base to leave an anion. Explain
briefly, then do Problem 16.60.
'
C
P
O
PROBLEM 16.58 Les Gometz, a professor from New York
who is very often disappointed in September, was interested
in investigating the Diels–Alder reaction of methyl coumalate
A and the enamine B. The professor attempted to prepare B by
allowing propionaldehyde and morpholine C to react.
OH
Na
2
Cr
2
O
7
H
2
SO
4
/H
2
O
35 ⬚C
O
O
P(Ph)
3
1. O
3
2. HOOH
A B C
CH
3
CH
+
+–
C
O
OH
O
COOCH
3
A
B
O
CH
3
CH
N
O
CH
?
HN
O
+
+
O
H
C
H
O
CH
2
R
O
CH
2
R
BH
-
-
..
..
B
+
H
O
CH
2
H
O
CH
2
KOH/H
2
O
H
OH
CH
3
HO
O
O
After an appropriate time, Professor Gometz assayed the
reaction mixture by
1
H NMR spectroscopy and was disap-
pointed to find that only a small portion (5–10%) of the
desired enamine B had formed. Ever the optimist, he ran the
Diels–Alder reaction with diene A anyway, using the reaction
mixture containing what he knew to be only a small amount
of B. He was delighted and astonished to obtain an 80%
yield of cycloadducts. Your task is to explain how Professor
Gometz could get such a good yield of products when only a
small amount of enamine B was present in the reaction
mixture. Hint: See Problem 16.9.
PROBLEM 16.55 Rationalize the following somewhat surpris-
ing result. Hint: This problem is not as difficult as it looks.
First, consider what the reaction of the alcohol starting material
with the chromium reagent will give. Second, what will happen
when this compound reacts with more alcohol? Finally, use the
chromium reagent again.
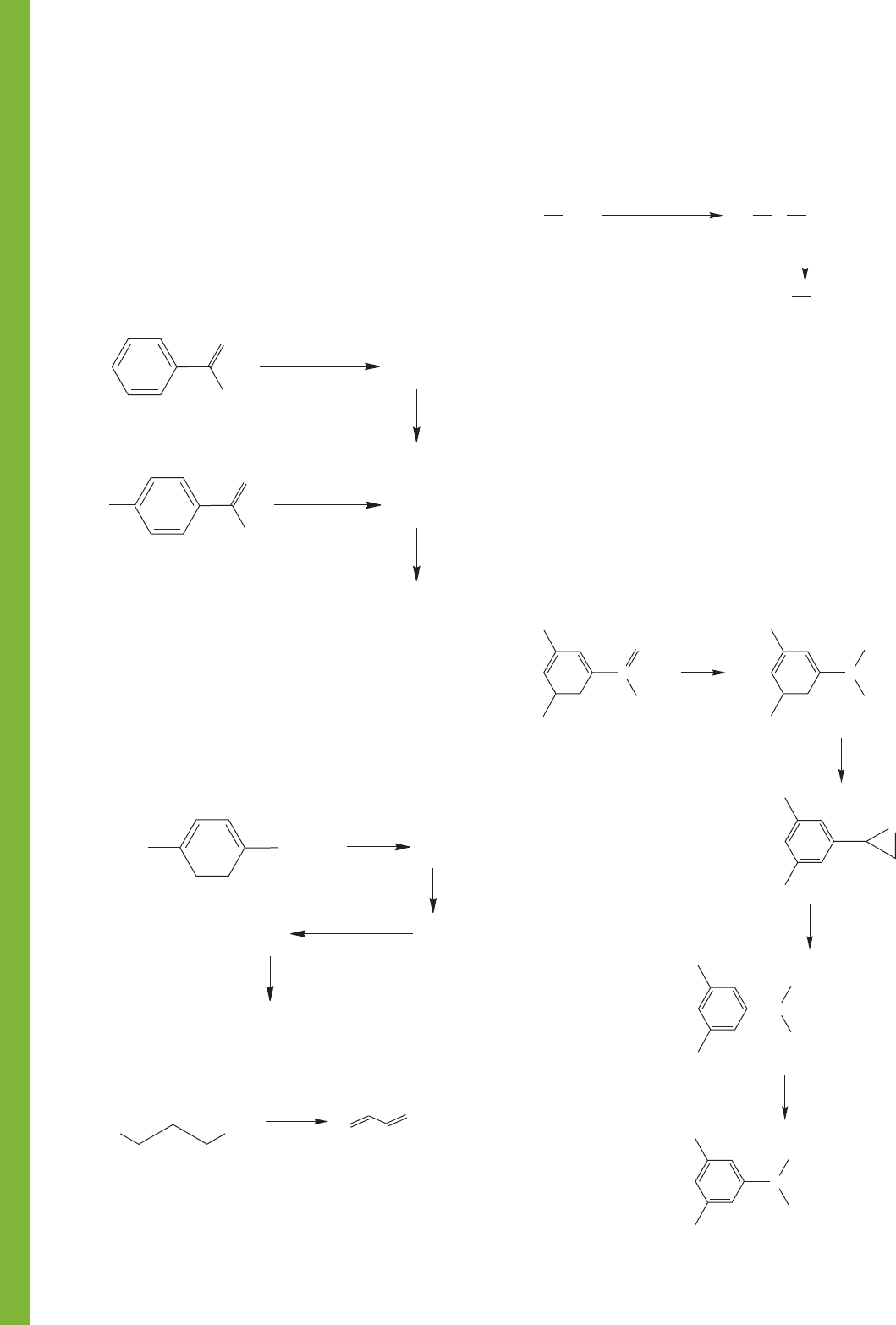
826 CHAPTER 16 Carbonyl Chemistry 1: Addition Reactions
PROBLEM 16.63 Provide structures for compounds A–D.
Spectral data for compound D are shown below. Although
mechanisms are not required, a mechanistic analysis may prove
helpful in deducing the structures.
Compound D
IR (neat): 2817 (w), 2717 (w), 1730 (s) cm
1
1
H NMR (CDCl
3
): δ 0.92 (t, J 7 Hz, 6H), 1.2–2.3 (m, 5H),
9.51 (d, J 2.5 Hz, 1H)
13
C NMR (CDCl
3
): δ 11.4, 21.5, 55.0, 205.0
PROBLEM 16.64 Write a mechanism for the acid-catalyzed
transformation of glycerol into acrolein.
THF, Δ
2. H
2
O/H
3
O
+
1. B, Δ
H
2
O/H
3
O
+
H
3
CO
O
Ph
C
D
H
3
O
+
HOCH
2
CH
2
OH
THF, Δ
Mg
Br
O
CH
3
A
B
H
2
O/H
3
O
+
Ph
3
P
OCH
2
ClH
3
C
A
PhLi
BC
D
—
(CH
3
CH
2
)
2
C—O
—
Ph
3
P—O
+
OH
Glycerol Acrolein
H
3
O
+
H
2
O
O
H
HO
HO
OH
1. NaH
2. CH
3
OCH
2
Cl
H
3
O
+
H
2
O
R
OHR
O CH
2
OCH
3
R
PROBLEM 16.66 Thiols are important participants in deter-
mining enzyme shapes and reactivity. Nature has selected for
this thiol involvement because the sulfur-sulfur bond of the
disulfide is formed reversibly. The reaction is
What is the name for the type of reaction that converts thiols
into disulfides? What type of reaction is the reverse of this
process? What extraordinarily common biological reagent will
convert a thiol into a disulfide? What biological reagent will
convert a disulfide into a thiol? Hint: see p. 814.
PROBLEM 16.67 Metaproterenol (orciprenaline, 1) is a
β-adrenoreceptor stimulant used therapeutically as a bron-
chodilator. In the synthesis of the hydrobromide salt of racemic
1, outlined below, supply the appropriate reagents.
RS
O
SR
Z——
—U
RSH + RSH
O
CH
2
Br
(a)
O
OH
CH
2
Br
(b)
(d)
Cl
–
(c)
OH
CH
2
NH
2
CH(CH
3
)
2
1
Br
–
+
CH
3
O
CH
3
O
CH
3
O
CH
3
O
CH
3
O
CH
3
O
CH
3
O
CH
3
O
C
CH
HO
HO
CH
OH
CH
2
NH
2
CH(CH
3
)
2
CH
+
PROBLEM 16.65 This problem introduces another alcohol
protecting group, methoxymethyl (MOM), particularly popular
in the month of May. The MOM protecting group is stable to
base, but it can be cleaved upon treatment with mild acid.
Provide an arrow formalism for the introduction of the MOM
protecting group. Also explain why this protecting group is
readily cleaved upon treatment with aqueous acid.
PROBLEM 16.62 Provide structures for compounds A–D.
Spectral data for compound D are shown below.
Compound D
Mass spectrum: m/z 332 (p, 82%), 255 (85%), 213 (100%),
147 (37%), 135 (43%), 106 (48%), 77 (25%), 43 (25%)
IR (KBr): 3455 (s) and 1655 (s) cm
1
1
H NMR (CDCl
3
): δ 2.58 (s, 3H), 2.85 (s, 1H, vanishes when a
drop of D
2
O is added), 3.74 (s, 3H), 6.77–7.98 (m, 13H)
13
C NMR (acetone-d
6
): δ 27.0, 55.8, 82.0, 128.1–159.9
(12 lines), 197.8
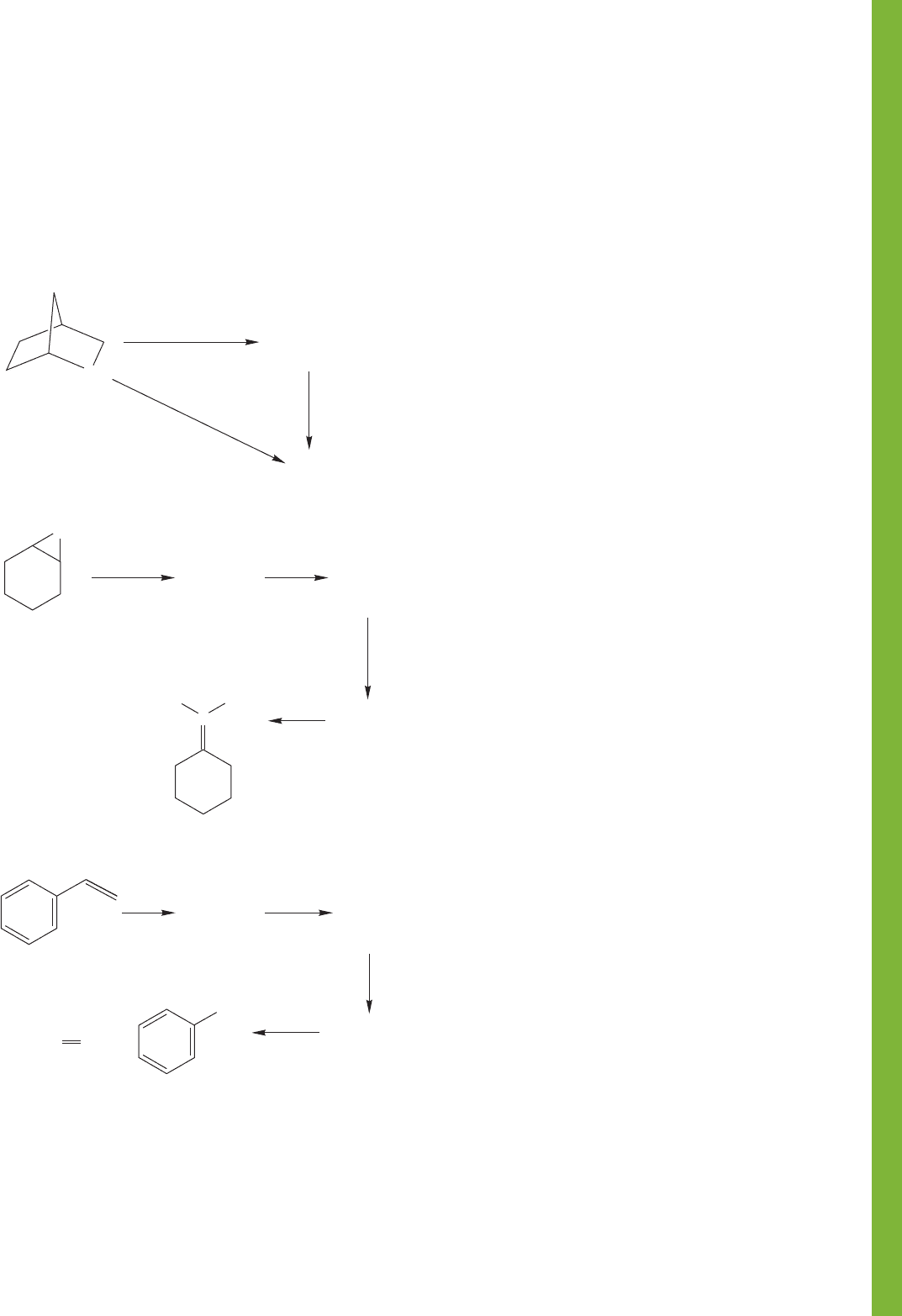
16.20 Additional Problems 827
PROBLEM 16.68
Treatment of 2-thiabicyclo[2.2.1]-heptane
(1) with 1 equivalent of hydrogen peroxide affords a mixture of
diastereomers 2 and 3 of molecular formula C
6
H
10
OS. Both
diastereomers 2 and 3 yield the same product 4 (C
6
H
10
O
2
S)
upon treatment with peracetic acid. Compound 4 is also pro-
duced when 1 reacts with 2 equivalents of peracetic acid.
Propose structures for compounds 2, 3, and 4. Hint: Start by
drawing a good Lewis structure for the starting material 1.
PROBLEM 16.69 Provide structures for compounds A–C.
Use Organic Reaction Animations (ORA) to answer the fol-
lowing questions:
PROBLEM 16.71 Select the “Carbonyl hydration” reaction and
view the animation several times. Notice that there are two
intermediates in the reaction. The first intermediate is protonat-
ed acetone and the second is the protonated gem-diol. The final
product in this reaction is higher in energy than the starting
material. Why is this the case? Provide a one-word description
for the first step in the reaction. Provide a one-word description
of the second step. Is this reaction reversible?
PROBLEM 16.72 View the “Acetal formation” reaction. There
are many intermediates in this reaction. How many? Which of
them is the most stable intermediate? Is the acetal or the ketone
favored in this reaction? Explain why. Remember that every
step in this reaction is reversible. If you want to drive the reac-
tion to the acetal, what would you do?
PROBLEM 16.73 The “Imine formation” reaction shows the
methanamine attacking acetone in the first step. The alkoxide
intermediate is then protonated by an acidic ammonium species
in a proton transfer process. It is known that imine formation
occurs faster under slightly acidic conditions (pH 5). Why
not show the acetone being protonated in the first step as we
did in Carbonyl hydration and Acetal formation? Select the
LUMO track for this animation and examine this MO on ace-
tone by pausing the reaction at the beginning. There are several
important facts that are confirmed by this calculated image.
Which atom of the molecule has the most LUMO density?
Can you see the effect of hyperconjugation? Where? Why?
PROBLEM 16.74 This chapter also covered the reactions that
are animated in “Grignard reaction,” “Carbonyl reduction,”
“Alcohol oxidation,” “Wittig reaction,” and “Diol cleavage.”
Observe each of these reactions. Why is the Grignard reaction
so unusual? Why does the Wittig reaction start at such a high
energy?
'
H
2
O
2
CH
3
COOH, 25 ⬚C
S
2
1
+
4
3
CH
3
COOOH
2 equiv. CH
3
COOOH
CH
3
COOH, 50 ⬚C
CH
3
COOH
50 ⬚C
O
(C
6
H
12
O) (C
6
H
11
Cl)
CH
3
H
3
C
C
(C
9
H
18
O)
AB
C
1. LiAlH
4
PCl
3
2. H
2
O
1. Li
2. (CH
3
)
2
C
—
—
O
3. H
2
O
H
2
O
H
3
O
+
+
(C
8
H
9
BrO)
(C
8
H
8
O)
(C
8
H
10
O
2
)
A
B
C
H
2
C
Br
2
H
2
O
NaOH
H
2
O
H
3
O
+
H
2
O
HIO
4
H
2
SO
4
CHO
O
PROBLEM 16.70 Provide structures for compounds A–C.

Carboxylic Acids
828
17.1 Preview
17.2 Nomenclature and Properties
of Carboxylic Acids
17.3 Structure of Carboxylic Acids
17.4 Infrared and Nuclear Magnetic
Resonance Spectra of
Carboxylic Acids
17.5 Acidity and Basicity of
Carboxylic Acids
17.6 Syntheses of Carboxylic Acids
17.7 Reactions of Carboxylic Acids
17.8 Special Topic: Fatty Acids
17.9 Summary
17.10 Additional Problems
17
NONSTEROIDAL ANTIINFLAMMATORY DRUGS Most nonsteroidal antiinflammatory drugs
(NSAIDs) are carboxylic acids. Aspirin, ibuprofen, and naproxen are the three most
common NSAIDs. NSAIDs are frequently used to relieve muscle and back pain, which
improves our flexibility.
