Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

17.1 Preview 829
1
This quotation comes from a 1944 issue of Walt Disney’s Comics and Stories,“Donald Duck the Mad Chemist.”
It was discovered by Peter P. Gaspar, then a postdoctoral fellow at Caltech, now a professor at Washington
University.Those of us who work with CH
2
have still not reproduced Professor Duck’s experiment with osmot-
ic fog, but a few of us are still trying.
If I mix CH
2
with NH
4
and boil the atoms in osmotic fog, I should get
speckled nitrogen!
—DONALD DUCK
1
He’s talking chemical talk!
—HUEY
But he knows nothing
—DEWEY
About chemicals!
—LOUIE
17.1 Preview
In this chapter, we explore the properties and chemistry of carboxylic acids (gen-
eral formula ). As with other functional groups, we will begin by
learning the IUPAC naming system, and then move on to structure. Carboxylic
acids have a rich chemistry; they are both acids (hence the name) and bases, and
we saw in Chapter 16 how the carbonyl group is a locus of reactivity. Although
many new reactions will appear in this chapter, most important will be the emer-
gence of a general reaction mechanism, addition–elimination, that we have
already seen briefly in Chapter 14. Chapter 18 will extend the exploration of this
process.
R
O
COOH
WORKED PROBLEM 17.1 What are the reactions we have learned for making car-
boxylic acids?
ANSWER Carboxylic acids can be made from oxidative ozonolysis of alkenes, by
oxidation of primary alcohols using dichromate under aqueous conditions, and
by oxidation of aldehydes.
ESSENTIAL SKILLS AND DETAILS
1. This chapter’s centerpiece is the Fischer esterification–acid hydrolysis equilibrium. If
you know this pair of reactions well, you will have much of this chapter under control.
The two mechanisms are exactly the reverse of each other—one is the “forward”
reaction, the other is the “backward”reaction.
2. The reaction of a carboxylic acid with two equivalents of an alkyllithium reagent,
followed by hydrolysis, yields a ketone. This reaction is a very effective way to make
these carbonyl compounds. Be sure you understand why two equivalents of alkyllithium
are necessary.
3. The reaction of LiAlH
4
with a carboxylic acid results in formation of a primary alcohol
through addition of two equivalents of hydride.
830 CHAPTER 17 Carboxylic Acids
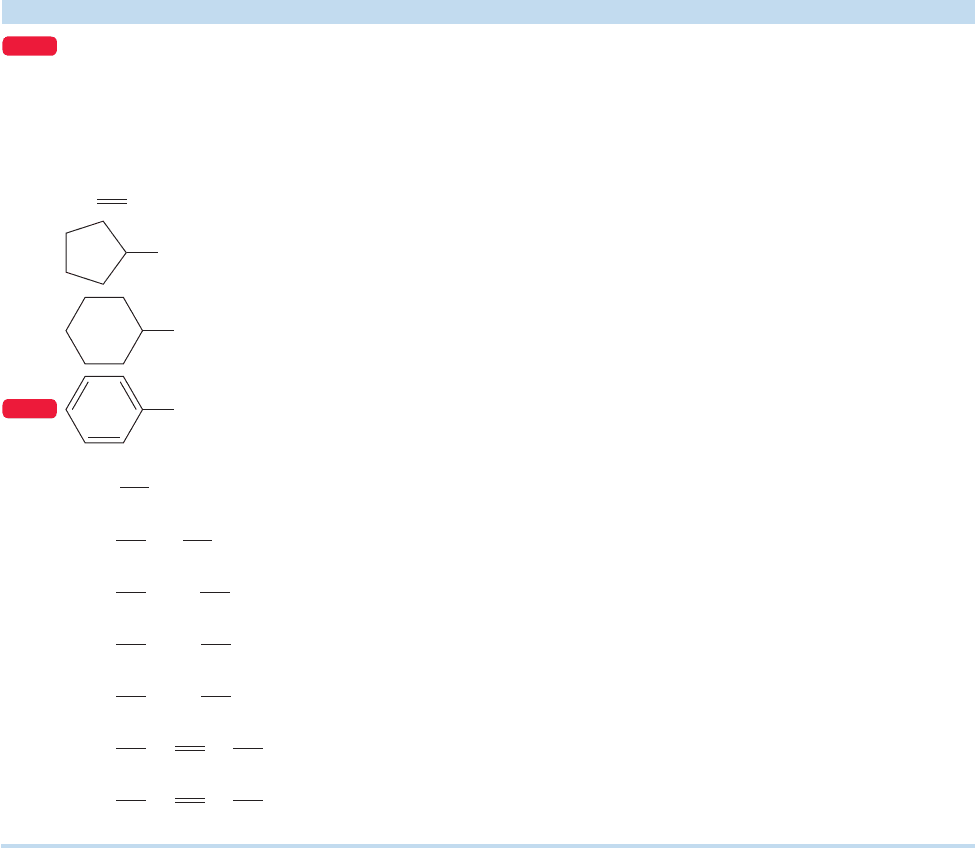
17.2 Nomenclature and Properties
of Carboxylic Acids
In the IUPAC systematic nomenclature, the final “e”of the parent alkane is dropped,
the suffix “oic” is added, and the separate word “acid” is added. Diacids are named
similarly, except that the suffix is “dioic,”and the final “e”is not dropped.Cyclic acids
are named as “cycloalkanecarboxylic acids.”Table 17.1 shows some common mono-
and diacids along with some of their physical properties and common names, many
of which are still used.
COOH
COOH
COOH
TABLE 17.1 Some Carboxylic Acids and eir Properties
Structure Systematic Name Common Name bp (°C)
HCOOH
CH
3
COOH
Methanoic acid Formic acid
Acetic acid
100.7
117.9Ethanoic acid
CH
3
CH
2
COOH
Propanoic acid Propionic acid 141
CH
3
CH
2
CH
2
COOH
Butanoic acid Butyric acid 165.5
CH
3
CH
2
CH
2
CH
2
COOH
CH
3
CH
2
CH
2
CH
2
CH
2
COOH
Pentanoic acid Valeric acid
Caproic acid
186
205Hexanoic acid
Propenoic acid Acrylic acid 141.6
Cyclopentanecarboxylic acid 216
mp (°C)
8.4
16.6
–20.8
–4.5
–33.8
–2
13
–7
pK
a
Cyclohexanecarboxylic acid 232 31
Benzenecarboxylic acid Benzoic acid 249 122
a
ese are the pK
a
values for loss of the second proton.
CH
2
CHCOOH
Ethanedioic acid Oxalic acid 190
HOOC
COOH
Propanedioic acid Malonic acid 136
HOOC
CH
2
COOH
HOOC
(
CH
2
)
2
COOH
HOOC
(
CH
2
)
3
COOH
HOOC
(
CH
2
)
4
COOH
HOOC
CH
CH
COOH
Butanedioic acid Succinic acid 188
Pentanedioic acid Glutaric acid ~300 99
Hexanedioic acid Adipic acid >300 156
cis-Butenedioic acid Maleic acid 140
trans-Butenedioic acid
cis Isomer
trans Isomer
Fumaric acid ~300
3.77
4.76
4.87
4.81
4.82
4.83
4.25
4.91
4.88
4.19
1.23
4.19
a
2.83
5.69
a
4.16
5.61
a
5.41
a
4.31
5.41
a
4.43
6.23
a
1.92
4.38
a
3.02
HOOC
CH
CH
COOH
WEB 3D
WEB 3D
For substituted acids, the numbering of the longest chain begins with the acid
carbon itself, which is given the number “1”(Fig. 17.1). In cyclic compounds it is the
carbon attached to the “COOH”that is carbon “1”in naming.Because carboxylic acids
are the highest priority functional group, all functional groups except alkenes and
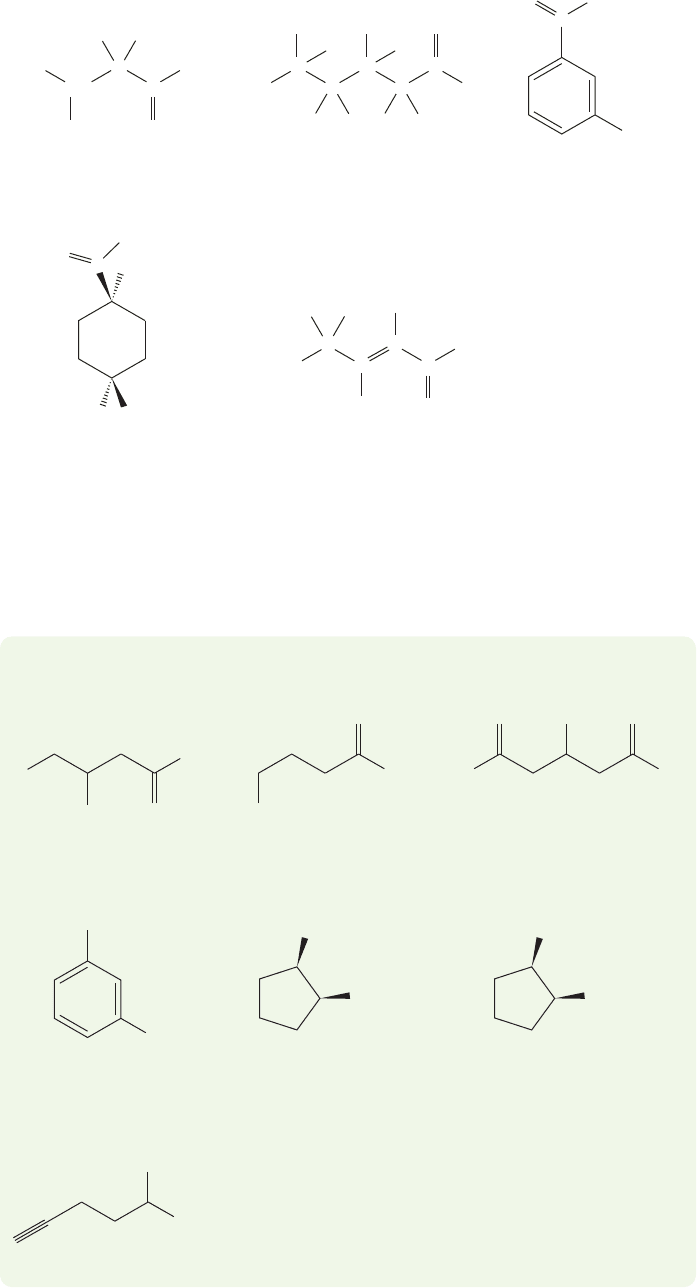
17.2 Nomenclature and Properties of Carboxylic Acids 831
HO
O
O
OH
(c)
OH
O
NH
2
(b)
OH
O
(a)
This enantiomer
Br
COOH
(f)
Br
(racemic)
COOH
(e)
COOH
NO
2
(d)
OH
CO
2
H
(g)
..
..
..
..
O
..
OH
..
..
OH
..
Br
..
CH C
H
3
C
3-Bromobutanoic acid
(3-bromobutyric acid)
3,5-Dichlorohexanoic acid 3-Ethylbenzoic acid
(m-ethylbenzoic acid)
cis-4-Aminocyclohexane-
carboxylic acid
C
C
O
..
..
..
..
..
..
CC
OHH
3
C
C
Cl
..
..
..
Cl
..
..
O
..
..
..
..
..
..
..
..
O
..
..
H
H
NH
2
C
..
..
OH
C
1
2
3
5
6
42
3
1
HH
HHO
CC
O
H
H
OH
C
H
3
CC
H
H
H
H
C
HH
(E )-4-Hydroxy-2-pentenoic acid
CH
2
CH
3
FIGURE 17.1 Some carboxylic acids
and their names.
alkynes are listed as substituents in the prefix. Alkenes and alkynes are indicated in
the parent name. You will need to be able to recognize the condensed formula for a
carboxylic acid, which is RCOOH, often written as RCO
2
H.
PROBLEM 17.2 Name the following compounds:
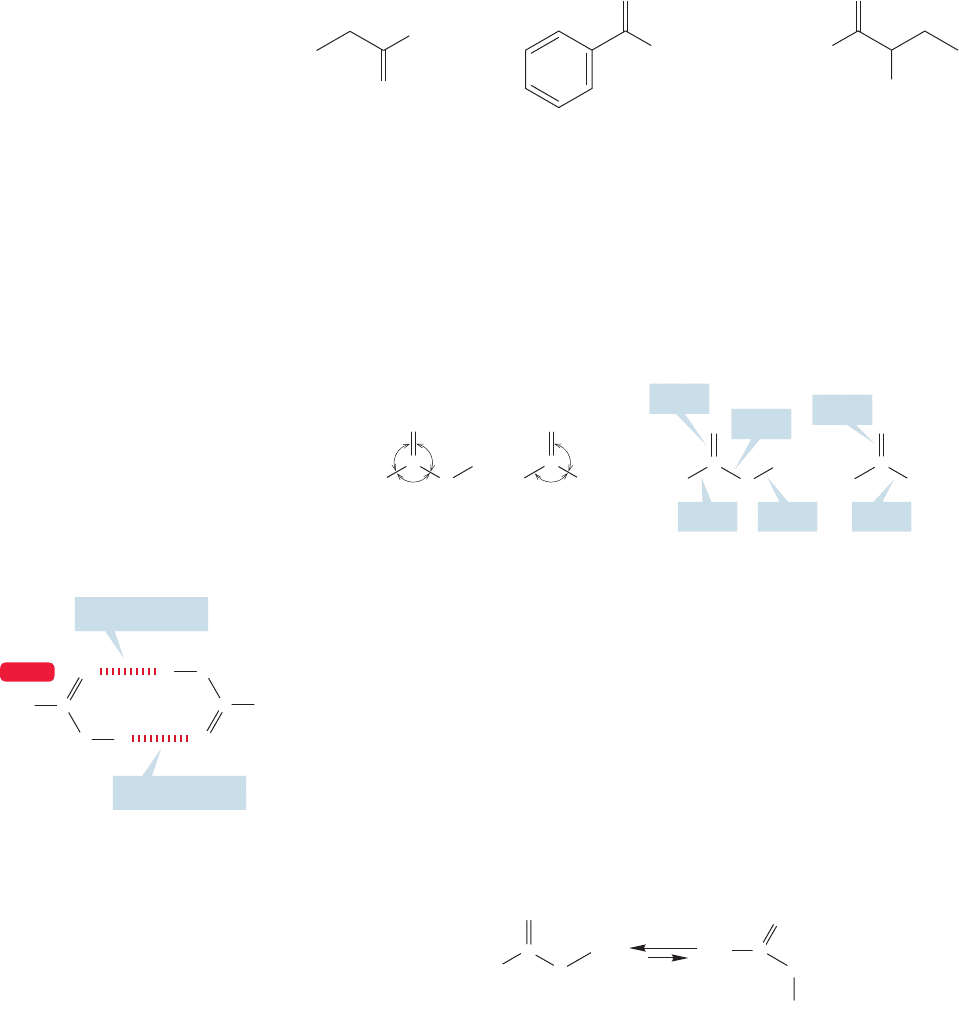
832 CHAPTER 17 Carboxylic Acids
Lithium benzoate Potassium 2-chlorobutanoateSodium propanoate
O
–
O
Na
+
O
–
Li
+
O
Cl
–
OK
+
O
FIGURE 17.2 Some carboxylate salts
and their names.
C
H
H
O
O
125⬚
111⬚
Bond angles
124⬚
C
H
O
H
121⬚
118⬚
C
H
H
O
O
Bond lengths
C
H
O
H
1.10
1.32 A
⬚
1.23
1.23
0.97 1.11
A
⬚
A
⬚
A
⬚
A
⬚
A
⬚
FIGURE 17.3 The structure of formic
acid compared to that of
formaldehyde.
A carboxylate anion is the conjugate base of a carboxylic acid. Carboxylate anions
are encountered frequently in any study of organic chemistry.They are also often found
listed as contents of soaps and shampoos. So, it is worth learning the IUPAC system
for naming the carboxylates. We refer to RCOO
and its counterion (Li
,Na
,or
K
, for example) as a salt because the RCOO
M
is a result of an acidic hydrogen
replaced by a metal, which is the definition of a salt. The salt is named as if it were a
carboxylic acid, the suffix being changed from –ic acid to –ate.The name of the metal
counterion is added before the parent organic name as a separate word (Fig. 17.2). An
important property of carboxylate salts is that they are often soluble in water.
17.3 Structure of Carboxylic Acids
In Figure 17.3 we compare the structure of formic acid and the structure of formalde-
hyde.The structure of acids is essentially what one would expect by analogy to other
carbonyl compounds. The carbonyl carbons are sp
2
hybridized, and therefore
carbonyl compounds are planar.The strong carbon–oxygen double bonds are appro-
priately short, about 1.23 Å.
There are two complications with the structural picture. First, in solution the
simplest carboxylic acids are substantially dimerized (Fig.17.4).It is possible to form
two rather strong hydrogen bonds ( 7 kcal/mol, each) in the dimeric form, and this
energy gain accounts for the ease of dimer formation. In turn, the ease of dimer
formation helps explain the high boiling points (Table 17.1) of carboxylic acids.
Second, there are two energy minima for simple carboxylic acids formed by rota-
tion around the bond between the carbonyl carbon and OH oxygen. The
hydroxylic hydrogen can be anti to the carbonyl group, or eclipse it. We refer to these
conformations as s-trans and s-cis, respectively (p. 523) as shown in Figure 17.5.
These conformations are real energy minima separated in the gas phase by a barrier
of about 13 kcal/mol.The s-cis form for formic acid is more stable by about 6 kcal/mol.
C
O
O
'
Hydrogen bond
Hydrogen bond
H
H
O
O
O
C
CR
R
O
..
..
..
..
..
..
..
..
WEB 3D
FIGURE 17.4 A dimeric carboxylic acid.
H
O
..
..
H
H
s-cis s-trans
H
O
O
C
C
O
..
..
..
..
..
..
FIGURE 17.5 The s-cis and s-trans
forms of a simple carboxylic acid.
17.4 Infrared and Nuclear Magnetic Resonance Spectra of Carboxylic Acids 833
syn
O
..
..
..
..
..
..
..
O
O
H
CH
..
..
..
O
O
H
C
H
O
H
..
anti
H
H H
+
+
..
..
O
O
H
CH
H
+
..
..
..
..
O
O
H
C
H
HH
PROBLEM 17.3 Explain why the s-cis form of the carboxylic acid is more stable
than the s-trans form.
PROBLEM 17.4 Calculate the equilibrium constant for the equilibrium between
s-cis and s-trans formic acid at 25 °C.
WORKED PROBLEM 17.5 Interconversion of the s-cis and s-trans forms can occur
very easily in acidic water solution. Devise a mechanism not involving simple
rotation about a bond.
ANSWER A series of protonations and deprotonations will do the job.
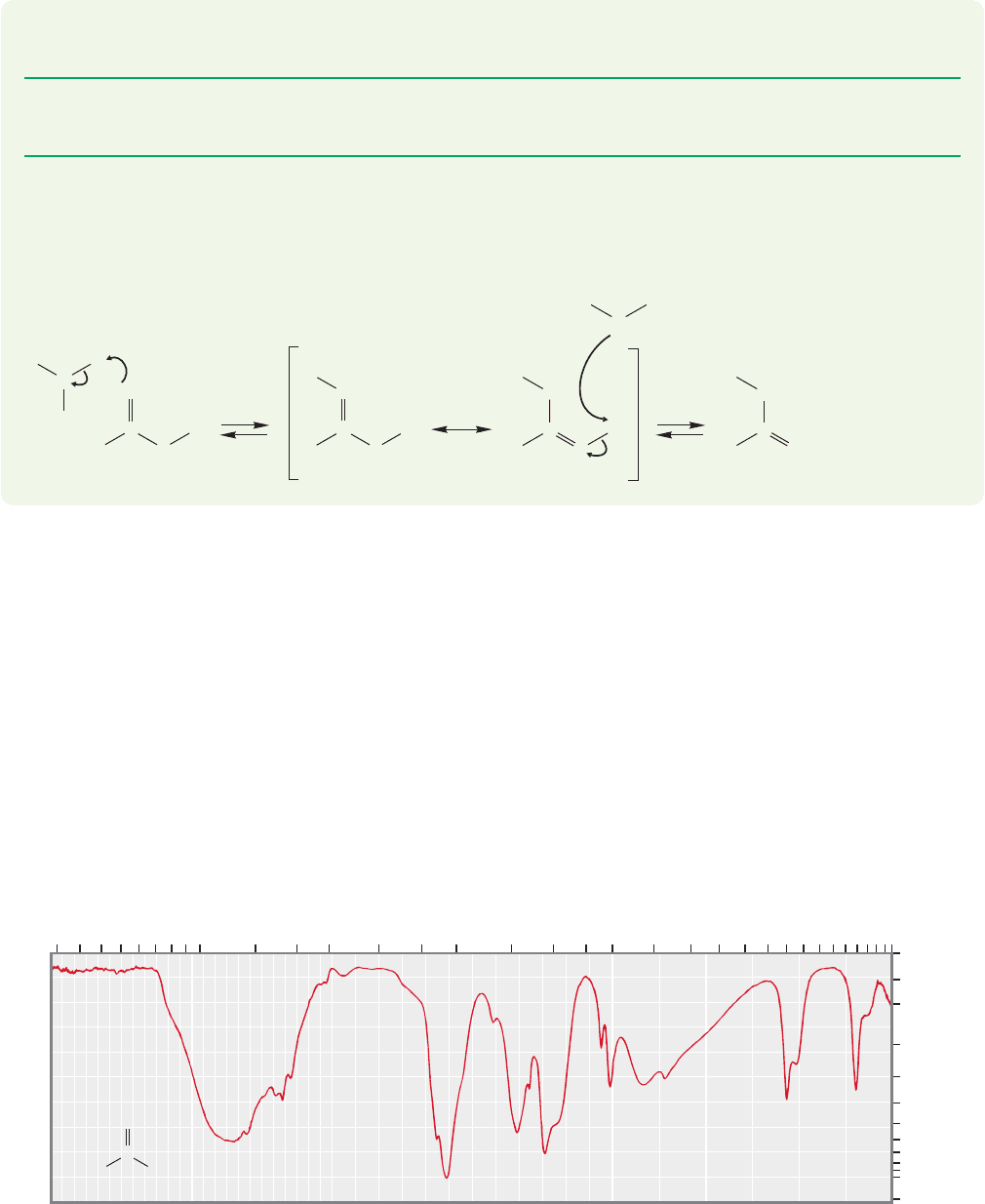
17.4 Infrared and Nuclear Magnetic Resonance
Spectra of Carboxylic Acids
The most prominent features of the IR spectra of carboxylic acids are the strong
absorption for the stretch at about 1710 cm
1
and the strong and very broad
band centered at 3100 cm
1
for the stretching frequencies.Figure 17.6 shows
the IR spectrum of acetic acid. As usual, conjugation of the carbonyl shifts the
stretch to a lower frequency by about 20 cm
1
.The stretching band
is broad for the same reasons that the stretching bands of alcohols are broad
(p. 710). There are many hydrogen-bonded dimers and oligomers with different
bond strengths present in any carboxylic acid sample.
The NMR spectra for a carboxylic acid are also informative. Hydrogens in
the position α to the carbonyl group of carboxylic acids are deshielded by both the
O
O
H
O
O
H
O
O
HC
P
O
O
O
H
C
P
O
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns ()
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
O
C
OH
H
3
C
FIGURE 17.6 The IR spectrum of acetic acid.
834 CHAPTER 17 Carboxylic Acids
electron-withdrawing nature of the carbonyl group and by local magnetic fields set
up by the circulation of electrons in the π bond.These hydrogens appear in the same
region as other hydrogens α to carbonyl groups at δ 2–2.7 ppm (see Table 15.4).
The acidic hydrogen of the RCOOH is the most deshielded hydrogen of all the typ-
ical organic functional groups. It usually appears in the region of δ 10–13 ppm as a
broad singlet. Like the hydrogen in the OH group of an alcohol, the hydrogen of a
carboxylic acid is exchangeable,and the signal vanishes when D
2
O is added to the sam-
ple and the OH becomes OD.Alcohol hydrogens rarely appear as low as δ 10–13 ppm.
The carbon of the carboxylic acid group is also strongly deshielded and appears down-
field,as do other carbonyl carbons in
13
C NMR spectra.The position is about 180 ppm,
slightly upfield of the chemical shifts of the carbonyl carbons of aldehydes and ketones.
17.5 Acidity and Basicity of Carboxylic Acids
Because carboxylic acids are both acids and bases, we might well expect that a rather
diverse chemistry would be found. Carboxylic acids wouldn’t be called acids if they
were not strong Brønsted acids. In fact, carboxylic acids are the organic functional
group that Nature uses as an acid, just as amines are the organic functional group
that Nature uses as its organic base. Table 17.1 gave the pK
a
values for some mono-
and dicarboxylic acids.
Organic acids (pK
a
3–5) are much stronger acids than alcohols (pK
a
15–17).
Reasons for this increased acidity are not hard to find, but assessing their relative
importance is more difficult. One explanation focuses on the formation of a
resonance-stabilized carboxylate anion after proton loss (Fig. 17.7).
H
Resonance-stabilized
carboxylate anion
O
O
CR
..
..
..
..
..
O
O
CR
..
..
..
..
..
O
O
CR
..
..
..
..
base
B
BH
–
–
+
..
–
WEB 3D
FIGURE 17.7 The formation of a
resonance-stabilized carboxylate
anion through removal of a proton
from the hydroxyl group of a
carboxylic acid.
Deprotonation of an alcohol gives an oxygen anion, an alkoxide, but the ion is
not stabilized by resonance (Fig. 17.8). It is reasonable to accept the notion that the
formation of the highly stabilized carboxylate is responsible for the high acidity of
carboxylic acids. Recently, however, this traditional view has been challenged by at
least three research groups with both theoretical and experimental arguments. In var-
ious ways, the research groups of Andrew Streitwieser (b. 1927) at Berkeley, Darrah
Thomas (b. 1932) at Oregon State, and Kenneth Wiberg (b. 1927) at Yale pointed
out the importance of the inductive effect of the highly polar carbonyl group. In
their view, the increased acidity of acetic acid over ethyl alcohol derives not from
Carboxylate,
resonance stabilized
O
OH
C
R
....
..
..
O
O
C
R
..
..
..
..
..
..
base
B
BH
–
..
base
B
–
–
(–)
+
H
Alkoxide, not
resonance stabilized
O
RCH
2
..
..
O
..
..
..
BH
–
+
RCH
2
FIGURE 17.8 A comparison of carboxylate and alkoxide anions.
17.5 Acidity and Basicity of Carboxylic Acids 835
resonance stabilization of the carboxylate anion, but from the electrostatic stabiliza-
tion afforded the developing negative charge by the adjacent polar carbonyl group
in which the carbon bears a partial positive charge (Fig. 17.9).They reckon that res-
onance could account for no more than about 15% of the total stabilization.
WORKED PROBLEM 17.6 Explain why fluoroacetic acid has a lower pK
a
than acetic
acid (2.66 vs. 4.76).
ANSWER The structure of the anion formed by removal of a proton tells the story.
The dipole in the carbon–fluorine bond stabilizes the fluoroacetate anion, which
makes removal of the acidic proton easier.
H
R
O
C
O
..
..
O
..
..
..
..
H
R
C
O
..
..
..
+
–
O
..
..
R
C
O
..
..
..
+
–
..
+
–
..
..
R
C
O
..
..
..
–
..
B
HB
–
O
FIGURE 17.9 Electrostatic
stabilization may account for the
acidity of carboxylic acids.The highly
polar carbonyl group stabilizes the
carboxylate anion.
Now we should ask why resonance might not be so important.The carboxylate anion
is surely delocalized,whereas the alkoxide ion is not.The key point is that the carbonyl
group of the acid is already so polar that little further delocalization can occur as the
anion is formed. The negative charge on the carbonyl oxygen and the positive charge
on the carbonyl carbon aren’t developed as the oxygen–hydrogen bond breaks; the
charges are already largely there! Both factors, the resonance stabilization of the car-
boxylate anion and the polarity of the carbonyl group, surely contribute to the acidity
of carboxylic acids.The question is only over their relative importance. We will use the
resonance stabilization argument,keeping in mind that there may be more to the story.
PROBLEM SOLVING
Watch out! Carboxylic acids are called acids because they are acidic. They will
deprotonate in base before they do any other reactions typical of compounds
containing carbonyl groups. “Everyone” forgets that seemingly simple fact, and
problem writers may try to trap you. Always remember: A carboxylic acid
deprotonates in base.
..
..
O
H
B
..
–
..
..
..
..
O
O
H
3
C
CH
The δ
+
stabilizes the carboxylate anion
B
..
–
O
..
..
O
H
3
C
C
..
..
..
–
O
..
..
..
–
..
..
O
H
2
C
C
F
..
..
..
δ
+
δ
–
..
..
O
H
2
C
C
A
F
..
..
..
δ
+
δ
–
836 CHAPTER 17 Carboxylic Acids
The stabilization by electron-withdrawing groups pointed out in Problem 17.6
is a general phenomenon. As Table 17.2 shows, acids bearing electron-withdrawing
groups are stronger acids than their parent compounds. The further the electron-
withdrawing group is from the acid, the smaller the effect on the pK
a
(Fig. 17.10).
TABLE 17.2 Acidities of Some Substituted
Carboxylic Acids
Acid pK
a
Acetic 4.76
α-Chloroacetic 2.86
α,α-Dichloroacetic 1.29
α,α,α-Trichloroacetic 0.65
α-Fluoroacetic 2.66
α,α-Difluoroacetic 1.24
α,α,α-Trifluoroacetic 0.25
α-Bromoacetic 2.86
α-Iodoacetic 3.12
α-Nitroacetic 1.68
pK
a
= 4.81
Butanoic acid
..
..
..
Cl
..
..
pK
a
= 4.52
4-Chlorobutanoic acid
O
CH
2
CH
2
CH
2
OH
C
..
..
..
..
O
CH
3
CH
2
CH
2
OH
C
..
..
..
..
..
Cl
..
..
pK
a
= 2.84
2-Chlorobutanoic acid
O
CH
3
CH
2
CH
OH
C
..
..
..
..
..
Cl
..
..
pK
a
= 4.06
3-Chlorobutanoic acid
O
H
3
CCHCH
2
OH
C
..
..
FIGURE 17.10 The greater the distance (number of bonds) between an electron-withdrawing group, here
chlorine, and the point of ionization, the less effect an electron-withdrawing group has on the acidity.
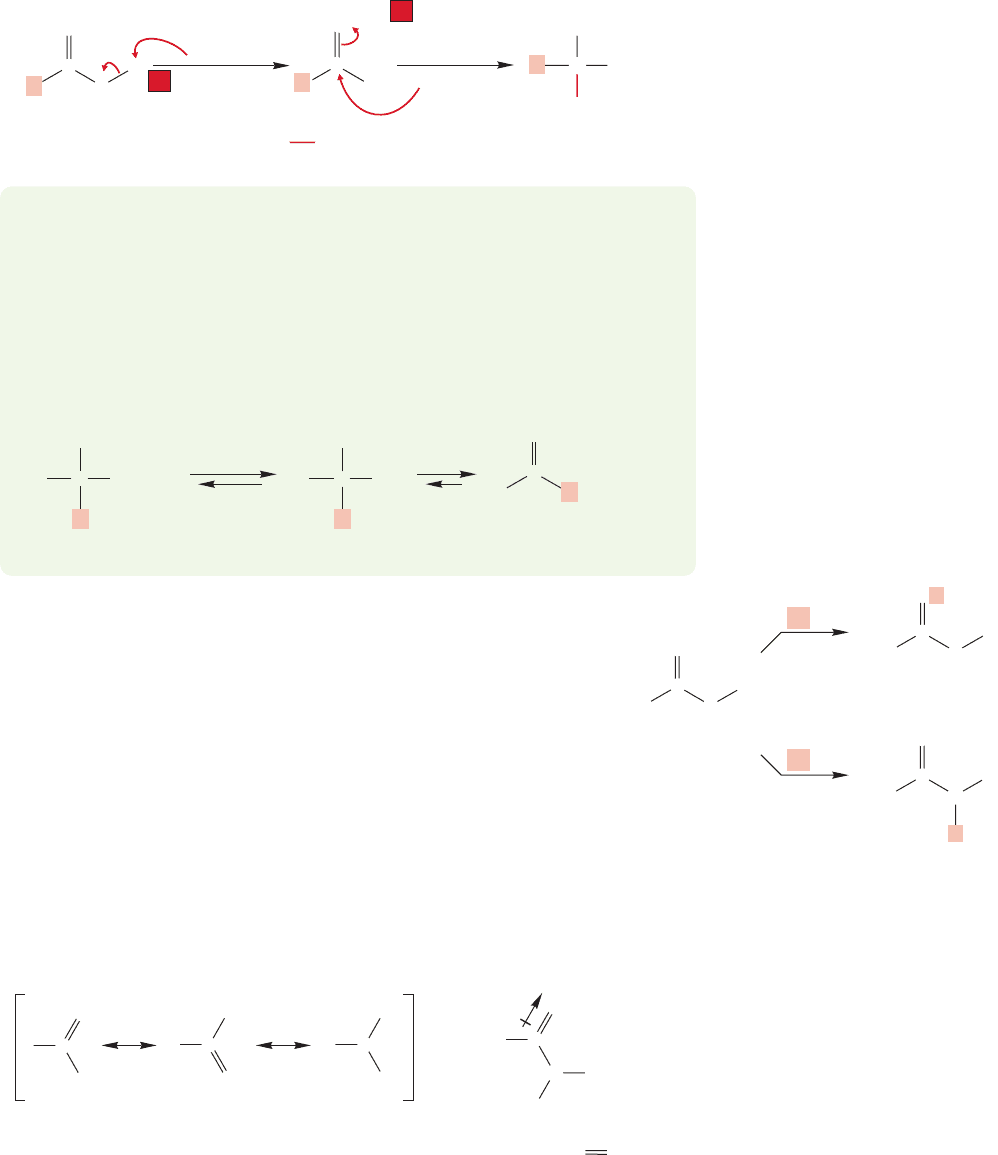
Carboxylic acids are electrophiles (Lewis acids) as well as Brønsted acids.
The presence of the carbonyl group ensures that. Remember all the addition reac-
tions of carbonyl groups encountered in Chapter 16. However, expression of the
Lewis acidity of the carbonyl carbon is often thwarted because the easiest reaction
with a nucleophile is not addition to the carbonyl group, but removal of the acid’s
OH hydrogen to give the carboxylate anion.Once it is formed, the carboxylate anion
is far more resistant to addition than an ordinary carbonyl because, in this case,
addition would introduce a second negative charge (Fig. 17.11).
H
base
(fast)
R
O
C
O
..
..
..
..
R
O
C
O
..
..
..
..
base
A dianion
(slow)
+
BH
..
–
R
O
C
..
..
..
..
..
..
–
–
B
..
B
–
..
B
–
O
FIGURE 17.11 For carboxylic acids, the fastest reaction with a nucleophile is removal
of the acidic hydroxyl hydrogen to give the carboxylate anion.The anion that results
is resistant to addition reactions with a second nucleophile, which would introduce
a second negative charge.
17.5 Acidity and Basicity of Carboxylic Acids 837
H
O
C
..
..
..
O
C
O
..
..
..
..
..
O
C
..
..
..
..
..
..
–
–
–
R
Li
+
+
Li
+
R
+
Li
..
R
–
+
Li
..
O
RH
Removal
of the acidic
hydrogen
O
..
Addition
to the
carbonyl
–
R
R
R
1
2
FIGURE 17.12 Some strong
nucleophiles can add to the
carboxylate anion. The organolithium
reagent is one example.
Finally, organic acids are nucleophiles (Lewis bases), and can react
with electrophiles (Lewis acids).The simplest reaction is the protona-
tion of a carboxylic acid. There are two possible sites for protonation,
the carbonyl oxygen and the hydroxyl oxygen (Fig. 17.13). Which will
it be?
The intermediate in which protonation has taken place at the car-
bonyl oxygen is resonance stabilized, whereas the species in which the
hydroxyl oxygen is protonated is not (Fig. 17.14). Moreover, protonation
of the hydroxyl oxygen is destabilized by the dipole in the carbon–oxy-
gen double bond,which places a partial positive charge on carbon. It will
not be energetically favorable to introduce a positive charge adjacent to
this already partially positive carbon. The more stable cation, in which
the carbonyl oxygen is protonated, is preferred.
Nevertheless, some especially strong nucleophiles are able to do this second addi-
tion. An example is the organolithium reagent,RLi, and shortly we will see the syn-
thetic consequences of this reaction. The formation of the dianion of Figure 17.12
is not as bad as it looks because the bond is substantially covalent. It is not
completely ionic.
O
O
Li
WORKED PROBLEM 17.7 Anticipate a little.What synthetic use can you see for the
reaction in Figure 17.12? Hint: What will happen when water is added once the
dianion is formed?
ANSWER When water is added, a hydrate will be formed. You know from
Chapter 16 that simple hydrates are generally unstable relative to a carbonyl com-
pound and water. So, this reaction should be a good synthesis of ketones. It is, as
we will see on page 856.
+
R
O
C
..
..
..
–
O
..
..
..
–
R
OH
OH
C
..
..
..
..
..
..
H
2
O
Hydrate
..
..
O
R
C
..
..
H
2
O
+
Li
+
Li
RR
R
H
or
O
C
Protonation of the
carbonyl oxygen
Protonation of the
hydroxyl oxygen
O
..
..
..
..
R
C
O
..
..
..
+
+
H
3
O
..
+
H
3
O
..
+
H
R
C
O
..
..
..
H
O
OH
H
R
FIGURE 17.13 The two possible sites for
protonation of a carboxylic acid.
C
R
C
R
C
R
O
C
Resonance stabilization of the product
of protonation of the carbonyl group
Unstabilized by resonance,
destabilized by the C
O dipole
O
..
..
..
..
..
..
+
OH
R
OH
..
..
..
..
+
+
OH
OH
..
..
..
+
OH
OH
H
H
δ
+
δ
–
FIGURE 17.14 Delocalization
stabilizes the intermediate resulting
from protonation of the carbonyl
oxygen.The carbon–oxygen dipole
destabilizes the cation formed from
protonation of the hydroxyl group.
838 CHAPTER 17 Carboxylic Acids
For these reasons, carboxylic acids are more strongly basic at the carbonyl oxygen
than at the hydroxyl oxygen. Does this mean that the hydroxyl group is never proto-
nated? Certainly not, but reaction at the more basic site is favored and will be faster.
PROBLEM 17.8 Explain why the thermodynamic stability of the protonated car-
bonyl should influence the rate (a kinetic parameter) of protonation.
With so many possible sites for reaction we might expect a rich chemistry of
carboxylic acids.That idea would be exactly right. Figure 17.15 summarizes the sites
of reactivity we have discussed so far.
H
R
O
C
O
..
..
..
..
Lewis basicity (greater)
(nucleophile)
Lewis basicity (lesser)
(nucleophile)
Lewis acidity
(electrophile)
Brønsted acidity
(electrophile)
FIGURE 17.15 The various sites of
reactivity for a carboxylic acid.
Summary
We have learned how to name carboxylic acids and their salts.The properties of
carboxylic acids include their acidity, their propensity to form dimers, the solu-
bility in water of the corresponding salt, their resonance stabilization, and their
multiple sites of reactivity.These traits make them useful reagents both in Nature
and the chemistry lab.
SALICYLIC ACID
myriad uses. It was synthesized as early as 1853, and its
analgesic properties were recognized by a group at
Farbenfabriken Baeyer in 1897. It apparently works by
inhibiting the production of an enzyme, prostaglandin
cyclooxygenase, that catalyzes the synthesis of molecules
called prostaglandins. Prostaglandins are active in many
ways, one of which is to help transmit pain signals across
synapses. No prostaglandins, no signal transmission; no sig-
nal transmission, no pain.
The simple aromatic carboxylic acid, salicylic acid, is a plant
hormone and is involved in many of the marvelously com-
plex interactions between plants and animals that makes the
study of biology so fascinating. Here’s a typical example
involving the voodoo lily. The flower of the voodoo lily emits
foul odors that attract flies, and the flies are used to transmit
pollen from the male reproductive organs to the female organs
of another lily. In the late afternoon, salicylic acid triggers
the first of two surges of heat, some 10–20 °C above normal.
This first surge releases the odor and attracts the flies, which
become trapped and coated with pollen. A second heat wave
the next morning opens the flower, awakens the pollen-
covered flies, which escape until the afternoon when they
are attracted to another voodoo lily and deposit the pollen.
Salicylic acid is also an analgesic. Indeed, the chewing of
willow bark, which contains salicylic acid, has been used to
control pain for thousands of years. You are probably most
familiar with salicylic acid in its acetylated form, acetyl sali-
cylic acid, or aspirin. Aspirin is a versatile pain killer and has
OH O
C
OH
Voodoo lily
Salicylic acid
