Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

17.6 Syntheses of Carboxylic Acids 839
17.6 Syntheses of Carboxylic Acids
We already know a number of routes to carboxylic acids,and this section will add
an important new one, the reaction of organometallic reagents with carbon
dioxide.
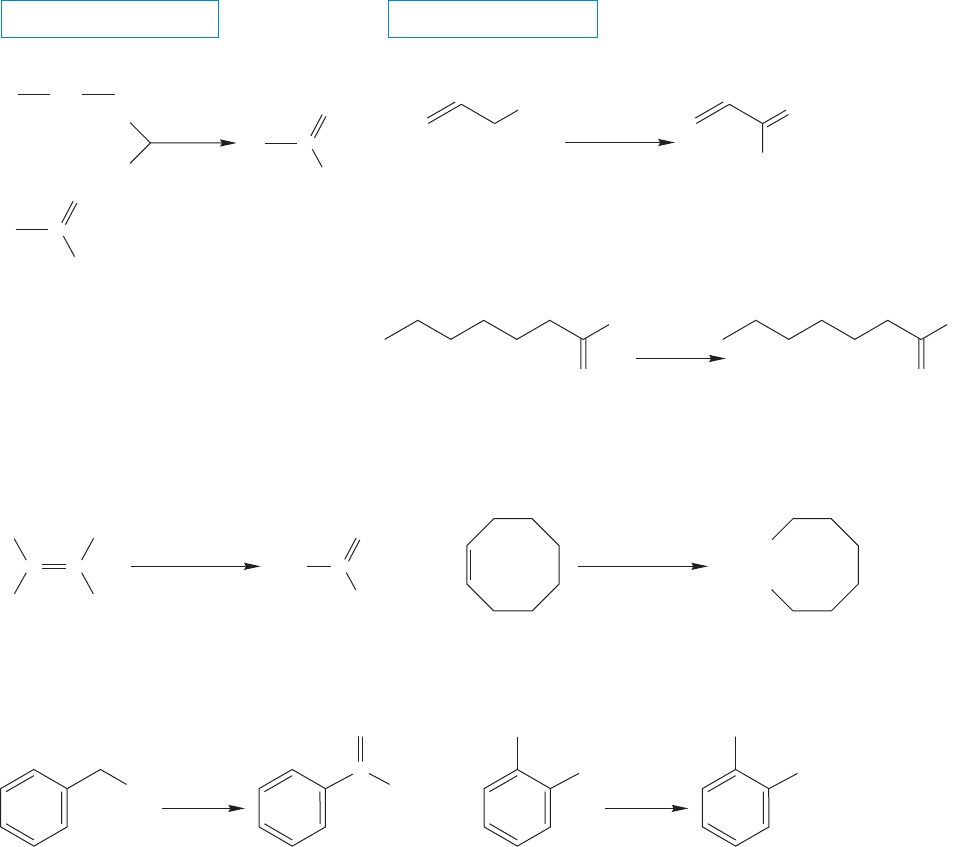
17.6a Oxidative Routes As you know, there are several oxidative reactions
that produce carboxylic acids (Fig. 17.16). Primary alcohols and aldehydes can
be oxidized to acids by a variety of oxidants including HNO
3
, KMnO
4
, CrO
3
,
K
2
Cr
2
O
7
, or RuO
4
(p. 802). In Figure 17.16, these reagents are generalized by
“[O]”, which stands for oxidant. Alkenes with a hydrogen attached to one or
both of the doubly bonded carbons can undergo ozonolysis using an oxidative
workup to give acids (p. 436). In addition, the side chains of alkyl aromatic com-
pounds can be oxidized to acid groups with KMnO
4
(p. 613).
CH
3
Cl
COOH
Cl
KMnO
4
(77%)
H
2
O
100 ⬚C
R
O
OH
C
KMnO
4
H
2
O
..
..
..
..
HOOC
HOOC
(63%)
1. O
3
2. HOOH/H
2
O
1. O
3
2. HOOH/H
2
O
CC
R
H
H
R
2 R C
OH
O
..
..
..
..
or
(100%)
RC
O
..
..
OH
..
..
OH
..
..
O
..
..
OH
..
..
H
2
CrO
4
CH
3
COOH
(77%)
KMnO
4
H
2
SO
4
[O]
RCH
2
OH
..
..
RC
H
O
..
..
..
..
O
H
..
..
..
O
OH
..
SPECIFIC EXAMPLES
THE GENERAL CASES
FIGURE 17.16 Oxidative routes to acids.
840 CHAPTER 17 Carboxylic Acids
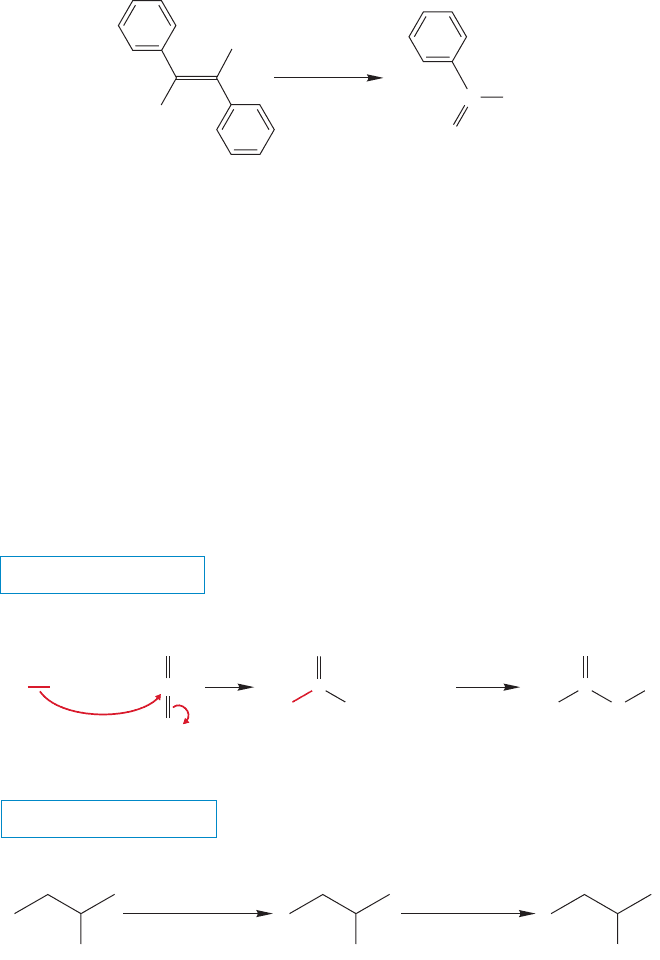
Alkenes can also be oxidatively cleaved by potassium permanganate to give car-
boxylic acids (Fig. 17.17). We already know that alkenes form vicinal diols when
treated with basic permanganate or OsO
4
(p. 443).The reaction with permanganate
can go further to form two acids. The trick is to use the polyether 18-crown-6
(p. 254) to make KMnO
4
soluble in benzene.The crown ether has a great ability to
bind potassium ions, and this property allows the negatively charged permanganate
ion (MnO
4
) to follow the bound cation K
(crown) into solution, where it is more
available for reaction with the organic substrate.
KMnO
4
2
18-crown-6
25 ⬚C
..
..
OH
..
..
O
C
H
H
(100%)
FIGURE 17.17 Another oxidative route to carboxylic
acids.
17.6b Reaction of Organometallic Reagents with Carbon Dioxide
Carboxylic acids can be obtained from the Grignard reaction. Like any other
compound containing a carbonyl group, carbon dioxide reacts with organometal-
lic reagents through an addition reaction (Fig. 17.18). The initial product is a
carboxylate salt that is converted into the acid when the reaction mixture is
acidified. This reaction is very general and a source of many acids. The typical
procedure for these reactions is simply addition of dry ice to the Grignard
reagent.
Mg
ether
Cl
..
..
..
MgCl
1. CO
2
, –12 ⬚C
2. 25% H
2
SO
4
..
..
..
COOH
(81%)
A SPECIFIC EXAMPLE
H
+
RMgX
..
..
..
..
O
..
..
O
O
(or RLi)
..
..
..
OMgX
R
C
C
–
..
..
O
..
..
O
R
C
+
..
..
H
2
O
H
3
O
..
+
THE GENERAL CASE
FIGURE 17.18 A general route to carboxylic acids uses the carboxylation of Grignard
reagents with carbon dioxide.
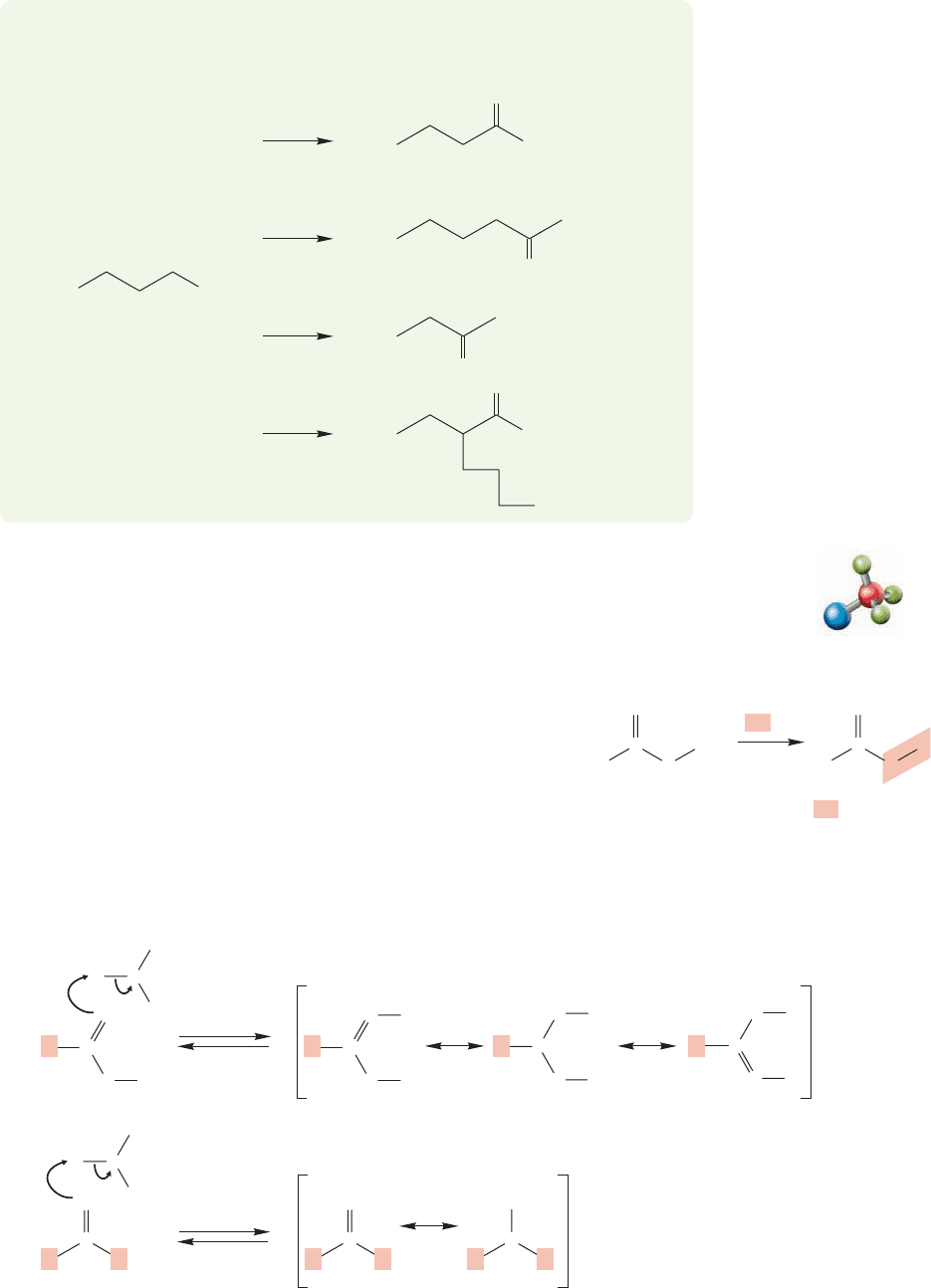
17.7 Reactions of Carboxylic Acids 841
OH
?
?
?
?
(a)
OH
O
(b)
OH
O
O
(c)
OH
(d)
OH
O
Protonation of the carbonyl oxygen of the carboxylic acid
..
+
+
H
H
H
H
H
ROH
..
+
H
2
OR
R
C
..
..
..
..
+
O
O
R
C
..
..
..
+
O
O
O
..
R
H
H
Protonation of the carbonyl oxygen of a ketone (from Chapter 16)
..
+
OH
..
..
+
OH
O
C
..
..
R
R
C
R
R
C
R
R
+
O
..
R
H
H
O
C
O
..
..
..
..
R
H
O
C
O
..
..
..
..
ROH
..
+
H
2
OR
..
..
R
H
(a)
(b)
FIGURE 17.20 (a) The first step in
the Fischer esterification mechanism
is protonation of the carbonyl oxygen
to give a resonance-stabilized
intermediate. (b) The analogous
process with an aldehyde or ketone.
17.7 Reactions of Carboxylic Acids
17.7a Formation of Esters: Fischer Esterification An ester is a derivative of
a carboxylic acid in which the hydrogen of the hydroxyl group has been replaced with
an R group. We will examine the nomenclature, properties, and chem-
istry of esters in detail in Chapter 18, but one of the most important
syntheses of these species involves the acid-catalyzed reaction of car-
boxylic acids with excess alcohol, and must be given here. The reaction
is called Fischer esterification after the great German chemist Emil
Fischer (1852–1919) (Fig. 17.19).
We can write the mechanism for this reaction rather easily,
because its important steps are quite analogous to reactions of other
carbonyl-containing compounds (Fig. 17.20). As in the reaction of
..
ROH
acid
R
C
O
..
..
..
..
..
HOH
..
..
O
..
R
C
O
..
..
..
O
H
R
+
An ester
(RO replaces HO)
FIGURE 17.19 Fischer esterification, the acid-
catalyzed reaction of a carboxylic acid with an
alcohol, yields an ester.
PROBLEM 17.9 Starting from inorganic reagents, 1-butanol,CO
2
,and tosyl chloride
as your only sources of carbon, devise syntheses of the following molecules:
Fischer esterification
842 CHAPTER 17 Carboxylic Acids
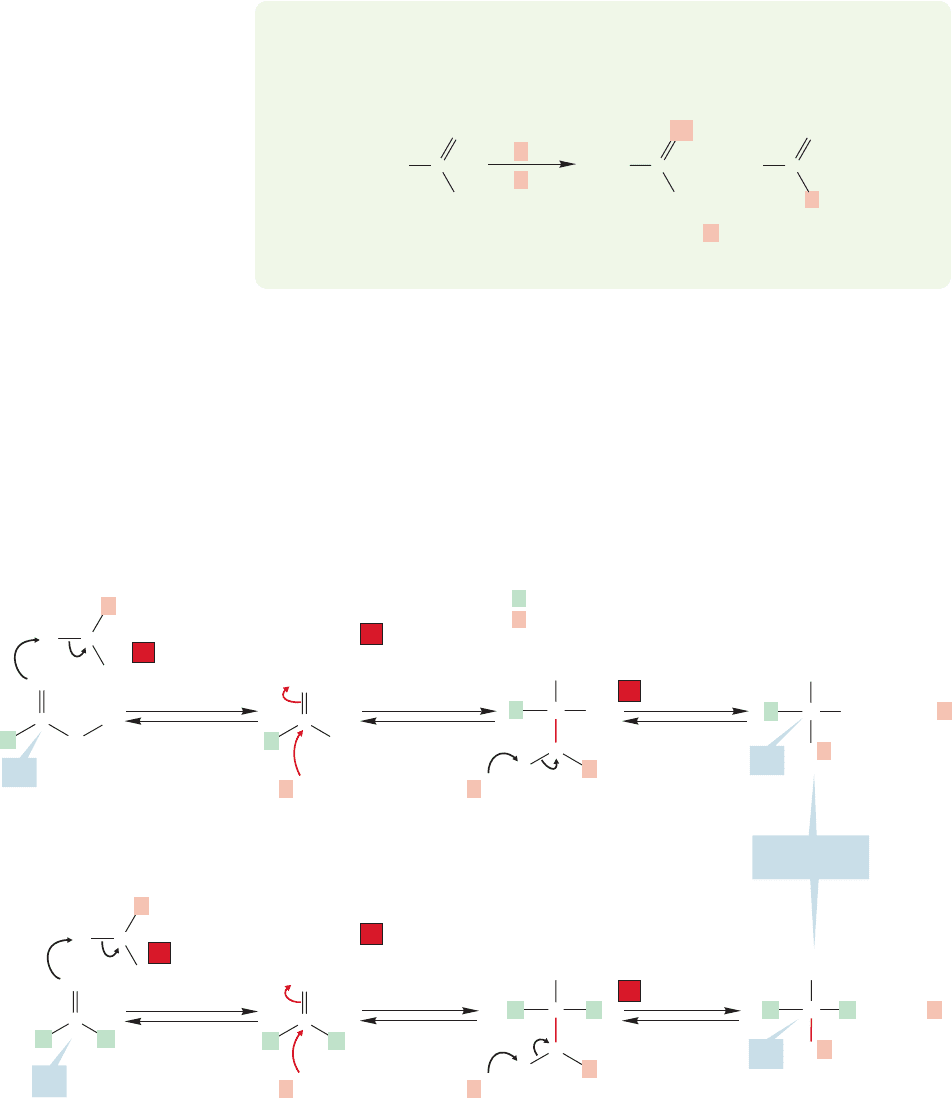
PROBLEM 17.10 Explain why treatment of acetic acid with leads to
exchange of both oxygen atoms. Red highlight indicates
18
O.
18
O
+
H
3
>
18
OH
2
..
18
OH
3
18
OH
2
H
3
C
OH
..
..
..
O
C
Labeled O appears
in both positions
+
H
3
C
OH
..
..
..
..
O
C
..
H
3
C+
OH
..
..
..
O
C
The second and third steps in Fischer esterification are also completely analo-
gous to parts of aldehyde or ketone chemistry. A molecule of alcohol acts as a nucleo-
phile and adds to the carbonyl carbon of the protonated carbonyl group (Fig. 17.21).
For a ketone, this addition is followed by deprotonation, and gives the hemiacetal;
for the acid, a somewhat more complicated intermediate with one more hydroxyl
group is formed. In both reactions, however, a planar, sp
2
hybridized carbon has been
converted into an intermediate with a tetrahedral carbon, called the tetrahedral
intermediate.
H
sp
2
O
C
O
..
..
..
..
C
OH
OH
..
..
..
..
..
+
+
+
C
..
..
..
..
..
O
+
O
..
H
H
H
..
+
H
2
OR
R
R
R
R
+
O
..
H
H
R
R
OH
Protonation
of the
carbonyl
Addition of
alcohol to the
protonated
carbonyl Deprotonation
Tetrahedral
intermediates
OH
C
..
..
..
..
R
OH
OH
ROH
..
..
ROH
..
..
OR
sp
2
sp
3
O
C
..
..
C
OH
..
..
..
+
+
+
C
..
..
..
O
H
R
R
R = Group on original carbonyl compound
R = Group on alcohol
..
+
H
2
OR
R
R
R
R
R
OH
ROH
..
..
ROH
C
..
..
..
..
OR
R
R
OH
sp
3
(b)
(a)
2
1
Protonation
of the
carbonyl
1
Addition of
alcohol to the
protonated
carbonyl
2
3
Deprotonation
Hemiacetal
3
FIGURE 17.21 (a) The second step in Fischer esterification involves addition of the alcohol to the protonated acid,
which is followed by deprotonation to give a tetrahedral intermediate. (b) The parallel process occurs with aldehydes or
ketones, in which case the hemiacetal is formed.
an aldehyde or ketone in acid, the first step is surely protonation of the carbonyl
group by the acid catalyst, to form a resonance-stabilized intermediate. Sulfuric
acid is often used as the acid catalyst.
17.7 Reactions of Carboxylic Acids 843
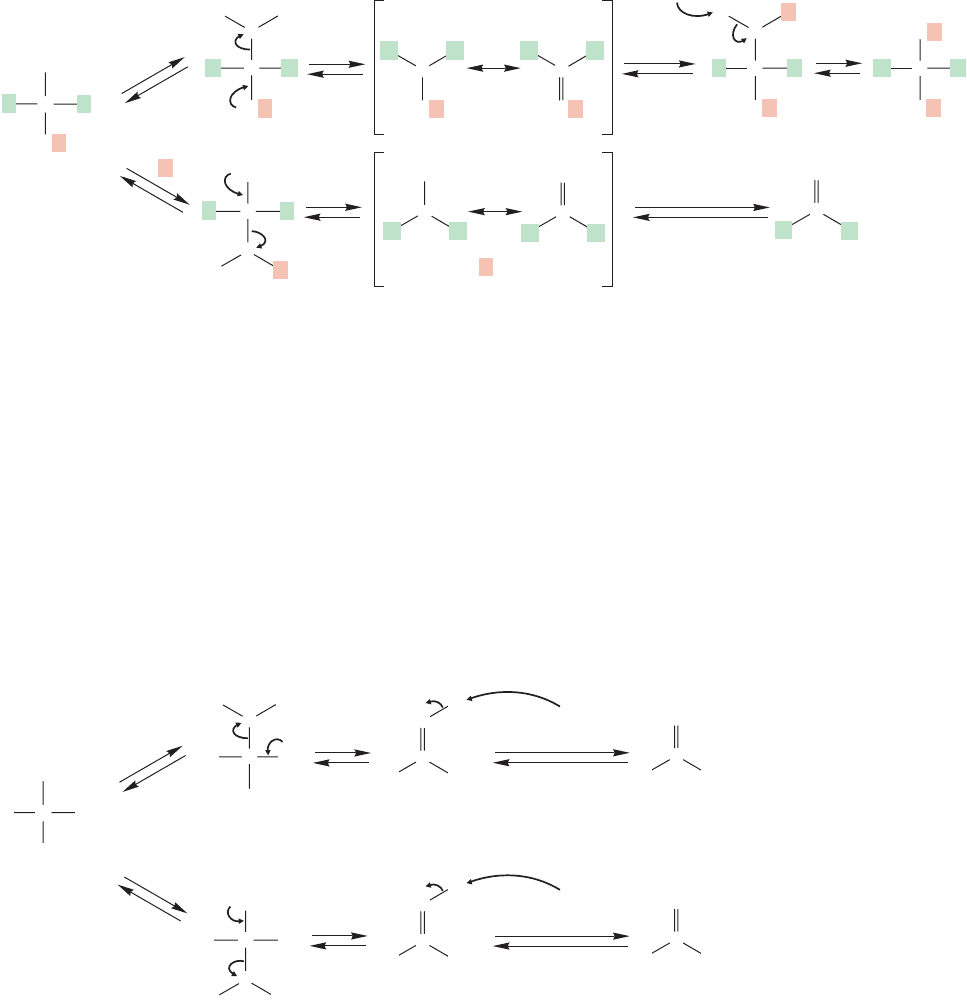
Now what can these tetrahedral intermediates do in acid? The hemiacetal
has only two options. It can reprotonate the OR group and then revert to the
starting ketone, or it can protonate the OH group and proceed to the full acetal
(Fig. 17.22).
+
+
..
..
H
2
O
protonate
OH
Hemiacetal
(tetrahedral
intermediate)
deprotonate
add
alcohol
Acetal
Ketone
C
..
..
..
OR
R
R
O
H
H
+
+
C
..
..
O
R
..
O
R
R
R
C
R R
C
..
..
..
O
H
protonate
OR
R
OH
C
..
..
..
..
..
O
R
R
R
R
C
..
..
R
..
..
R
R
R
O
H
+
C
..
..
..
..
O
R
R
R
OH
+
C
..
..
..
..
OH
R
R
+
C
..
OH
R
R
C
..
..
O
R
R
lose
water
lose
alcohol
ROH
ROH
O
O
R
R
FIGURE 17.22 For the hemiacetal intermediate, there are two further possibilities: Protonation of the OH
leads to water loss and formation of the full acetal, whereas protonation of the OR leads back to the original
starting ketone.
protonate
OH
Tetrahedral
intermediate
deprotonate
Carboxylic acid
C
..
..
..
OR
R
O
H
H
+
+
H
C
..
..
..
O
H
protonate
OR
R
OH
C
..
..
..
..
O
R
R
OH
OH
+
+
+
C
..
O
R
C
..
..
O
R
OH
lose
water
lose
alcohol
..
ROH
R
OH
..
..
OH
..
+
deprotonate
Ester
H
+
C
..
O
R
C
..
..
O
R
OR
..
..
..
ROH
..
ROH
2
+
..
ROH
2
..
OR
..
..
OH
..
..
..
..
..
..
FIGURE 17.23 For the tetrahedral intermediate from Figure 17.21a, there are also two further
possibilities: Protonation of the OR leads back to the starting acid, whereas protonation of either
of the OH groups leads to water loss and formation of the ester.
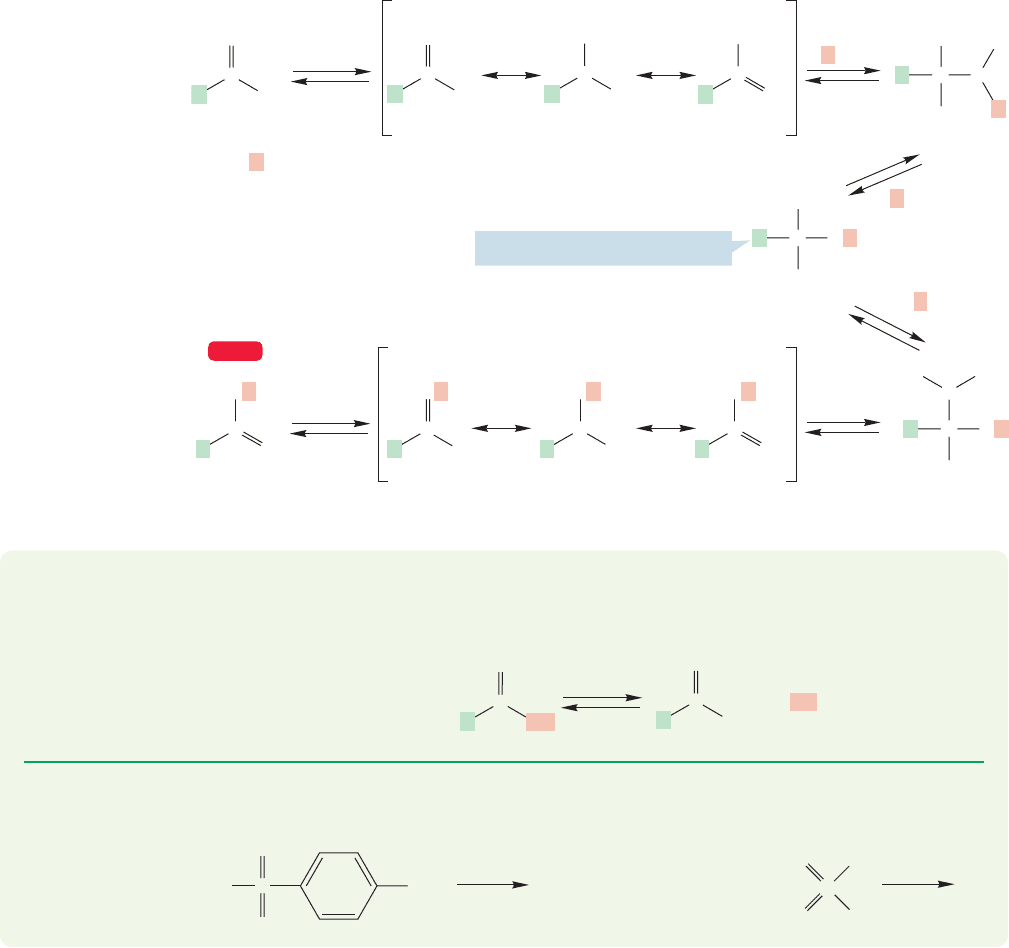
The tetrahedral intermediate formed from the acid has more options, because
it has one OR and two OH groups. Protonation of the OR simply takes us back
along the path to the starting acid. Protonation of one of the OH groups leads to
an intermediate that can lose water, deprotonate, and give the ester (Fig. 17.23). In
excess alcohol, the reaction is driven in this direction.
844 CHAPTER 17 Carboxylic Acids
PROBLEM 17.11 Write an arrow formalism mechanism, showing the electron-
pushing, for the acid-catalyzed formation of an organic acid from the reaction of
an ester with excess water.
..
..
..
..
+
C
..
..
OH
..
..
OH
R
+
+
+
C
..
R
C
R
C
..
..
O
OH
R
ROH
..
..
ROH
R
..
+
H
2
OR
..
..
+ H
2
O
Acid
..
..
OH
..
..
OH
..
OH
OH
+
+
..
C
..
..
..
..
..
OH
R
OH
O
BD
A
C
E
H
C
..
..
..
..
..
..
R
OH
OH
OR
C
..
..
..
OH
R
O
H
H
+
..
..
OR
C
..
..
O
R
C
..
..
OH
R
..
OR
..
OR
C
..
..
OH
R
..
..
OR
C
..
OH
R
..
..
OR
..
+
+
+ H
3
O
Ester
+
..
+
H
2
OR
Centerpoint of this mechanism
WEB 3D
FIGURE 17.24 The
full mechanism for
Fischer esterification
as well as for the
reverse reaction,
hydrolysis of an ester
to a carboxylic acid.
..
..
..
..
C
..
..
O
OH
R
ROH
..
..
H
2
O
C
..
..
O
R
..
..
OR
..
+
+
H
3
O
The complete mechanism for Fischer esterification is shown in Figure 17.24.
Note the symmetry of the process about the tetrahedral intermediate A. Note also
the similarity of B and C, the intermediates on either side of A. In intermediate B,
the OR is protonated. This pathway leads back to the starting carboxylic acid. In
intermediate C, it is an OH that is protonated. This path leads to the ester. To the
left of B and C are D and E, the two resonance-stabilized intermediates resulting
from protonation of the acid or ester carbonyl.
..
..
?
?
..
..
O
ROH
..
..
ROH
+
+
CH
3
S
..
..
O
..
..
..
..
..
..
..
O
Cr
..
O
OH
OH
Cl
..
..
..
PROBLEM 17.12 Ester formation is not new to you.Give products and mechanisms
for the following reactions, which appear in earlier chapters.
We have spent a lot of time and space on Fischer esterification and its reverse,
acid hydrolysis of the ester.We paid this close attention because this reaction is pro-
totypal and an anchor for further discussion.As for other anchor reactions (for exam-
ple, the S
N
2 reaction and addition of Br
2
to alkenes), knowing this reaction, quite
literally backward and forward, allows you to generalize and to organize the many
following reactions that show a similar pattern, although the details may be differ-
ent. Fischer esterification is definitely a reaction to know well.
17.7 Reactions of Carboxylic Acids 845
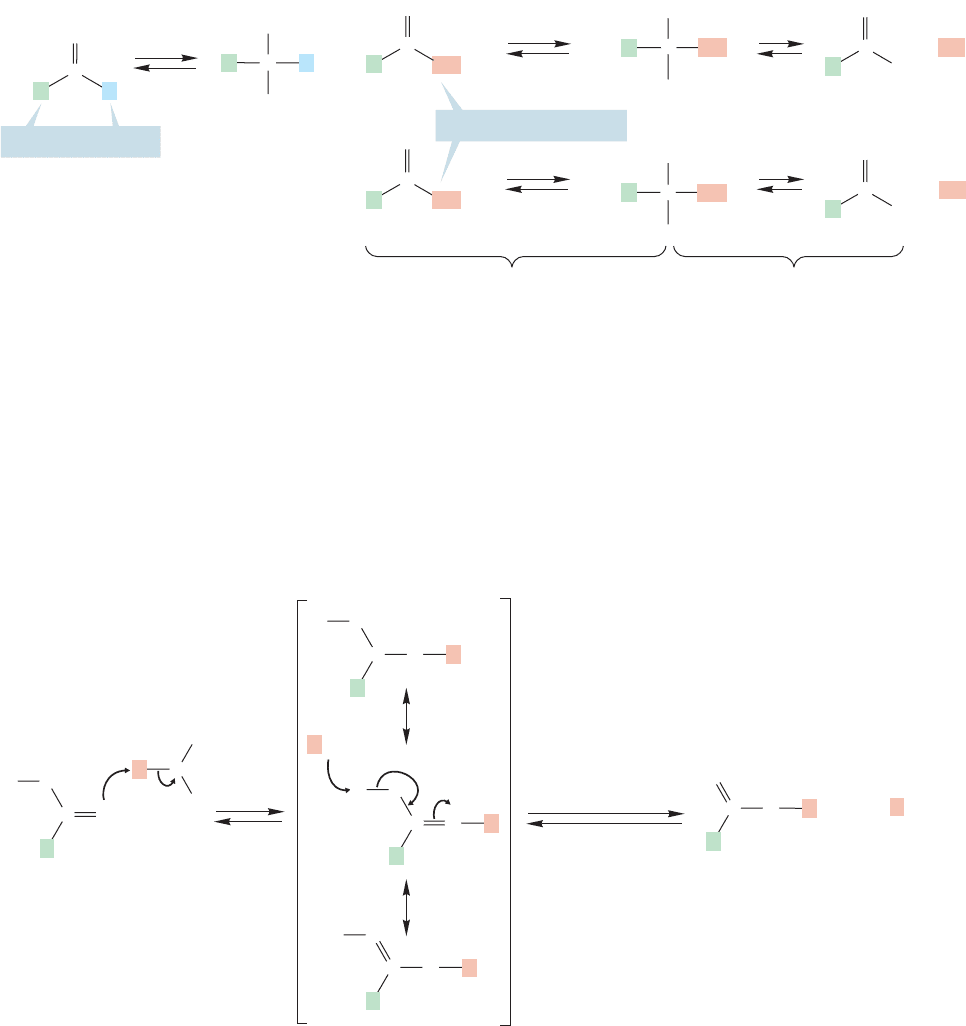
Note here what makes the chemistry of aldehydes and ketones different from that
of acids (or esters). In the aldehydes and ketones there is no leaving group present,
whereas in an acid (or ester) there is (Fig. 17.25). The chemistry of aldehydes and
ketones on the one hand, and the chemistry of acids and esters on the other, diverge
only after the nucleophilic addition of the alcohol to the carbonyl (see Fig. 17.21). In
the acids and esters there is a group that can be lost, providing a route to other mol-
ecules; in the aldehydes and ketones there isn’t, which means that the acids and esters
can undergo an addition–elimination reaction that the aldehydes and ketones can’t.
..
..
..
..
R
C
..
..
O
O
R
..
..
C
..
..
O
OH
R
ROH
C
..
..
O
R
R
C
..
..
O
R
B
HB
HB
HB
..
..
OR
C
..
..
R
B
..
..
OH
OH
C
R
B
C
..
..
R
B
OH
R
Potential leaving groups
Not a leaving group
Additions Eliminations
..
..
HOH+
C
..
..
O
R
B
+
..
..
OH
Protonated
alcohol
deprotonation
+
+
+
..
..
ROH
H
S
N
2
O
C
O
..
..
..
..
+
O
..
R
H
O
C
O
..
..
..
..
R
R
R
R
H
O
C
O
..
..
..
R
R
O
C
O
..
..
..
..
R
H
O
C
O
..
..
..
R
R
..
+
+
H
2
OR
H
H
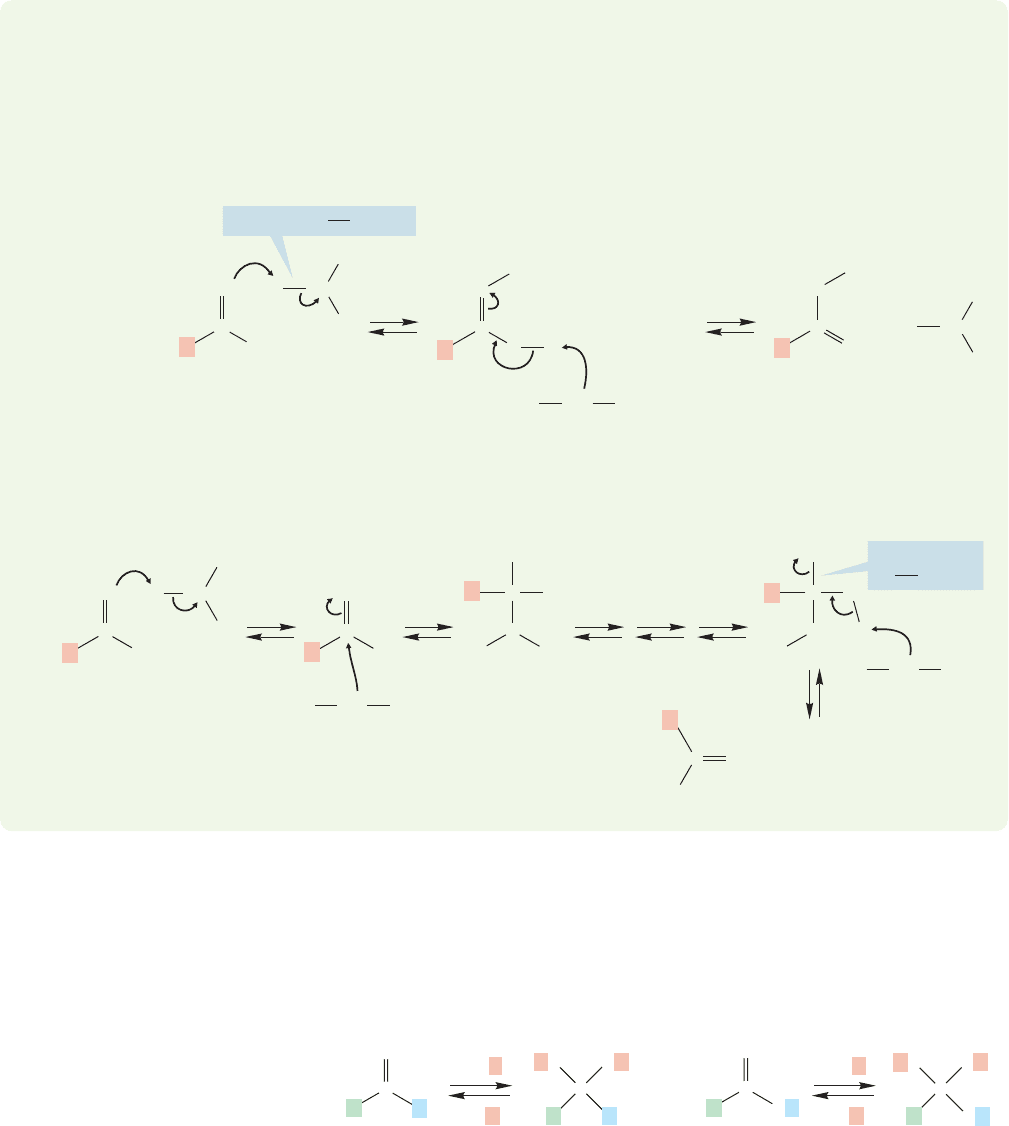
FIGURE 17.26 A potential mechanism for Fischer esterification. Here the acid is the nucleophile and the protonated
alcohol is the electrophile. Watch out for conventions! The figure shows proton removal from one resonance form,
which is purely for convenience sake—the resonance form has no individual existence.
But we should wait a moment. Mechanisms other than the one shown in Figure
17.24 for Fischer esterification can be imagined, and it is not fair to dismiss them
without evidence. For example, why not use the protonated alcohol as the electrophile
and the carbonyl oxygen of the carboxylic acid as the nucleophile rather than the
protonated carboxylic acid as the electrophile and the alcohol as the nucleophile?
Displacement of water using the acid as nucleophile could give the ester (Fig. 17.26).
FIGURE 17.25 Unlike aldehydes and ketones, acids and esters bear OH and OR groups that are
potential leaving groups.
846 CHAPTER 17 Carboxylic Acids
The key difference between the mechanism for Fischer esterification shown
in Figure 17.24 (which is correct) and this hypothetical one in Figure 17.26 is the
site of carbon–oxygen bond breaking. In the first (correct) mechanism, it is a
carbon–oxygen bond in the carboxylic acid that is broken; in the new (incorrect)
mechanism, it is the carbon–oxygen bond in the alcohol that breaks.
WORKED PROBLEM 17.13 Design an experiment to tell these two mechanisms (Fig.
17.24 and Fig. 17.26) apart. Assume you have access to any isotopically labeled
compounds you may need.
ANSWER Using labeled alcohol will do the trick. If the mechanism involved
carbon–oxygen bond breaking in the alcohol (it does not), the use of
18
O-labeled
alcohol would not lead to
18
O incorporation in the product ester.
However, if the mechanism involves breaking of a carbon–oxygen bond in the
original acid (it does)
18
O will be incorporated, which it is.
Break this
C
O bond
..
..
..
..
O
OH
C
H
..
..
..
OH
OH
C
18
O
..
..
H
R
+
+
protonations and
deprotonations
..
18
O
..
..
H
R
R
H
18
O
H
R
..
18
O
+
CO
R
18
O
++
R
18
OH
2
..
OH
OH
C
..
..
..
..
..
+
+
H
R
18
O
OH
2
O
C
..
..
..
..
..
..
..
..
..
..
H
2
O
R
R
R
R
R
Why don’t esters react further under these conditions? Aldehydes and ketones
form acetals when treated with an acid catalyst and excess alcohol.Why don’t esters
go on to form ortho esters (Fig. 17.27)? Ortho ester is the rather misleading name
used for the functional group that has the general structure of RC(OR)
3
.Ortho esters
are not real esters.
C
..
..
O
R
R
C
..
..
R
..
..
R
OR
RO
C
..
..
O
R
..
..
HOR
C
..
..
..
R
An ortho
ester
An acetal An esterA ketone
..
..
OR
..
OR
..
..
OR
RO
..
+
H
2
OR
..
..
HOR
..
+
H
2
OR
FIGURE 17.27 The conversion of an
ester into an ortho ester is analogous
to the formation of acetals from
aldehydes and ketones.
Acid
..
..
..
..
O
OH
C
R
..
..
..
O
O
C
H
18
O
..
..
H
R
R
+
Ester
..
..
..
..
O
O
C
R
H
H
..
18
O
+
R
H
H
..
18
O
+
Break this C O bond
++
H
18
OH
..
..
R
R
R
17.7 Reactions of Carboxylic Acids 847
Ortho ester
C
..
..
O
R
protonation addition
addition
deprotonation
proton
transfers
lose
water
C
..
R
OR
..
..
OR
..
..
OR
C
..
R
..
..
..
OR
..
+
H
2
OR
..
..
+
C
..
..
OH
R
+
+
+
C
..
OH
R
C
..
OH
R
ROH
..
..
OR
..
..
OR
..
..
OR
C
..
..
..
..
O
R
R
R
OH
O
H
..
+
C
..
..
..
..
OR
R
R
OR
O
H
..
C
..
..
..
OR
R
O
H
H
+
+
(+)
(+)
..
..
OR
OR
..
..
OR
..
..
HOR
..
..
HOR
..
..
HOR
..
Ester
FIGURE 17.28 A reasonable
mechanism for the conversion of an
ester into an ortho ester.
to those for acetal formation. However, stable ortho esters cannot be made this way.
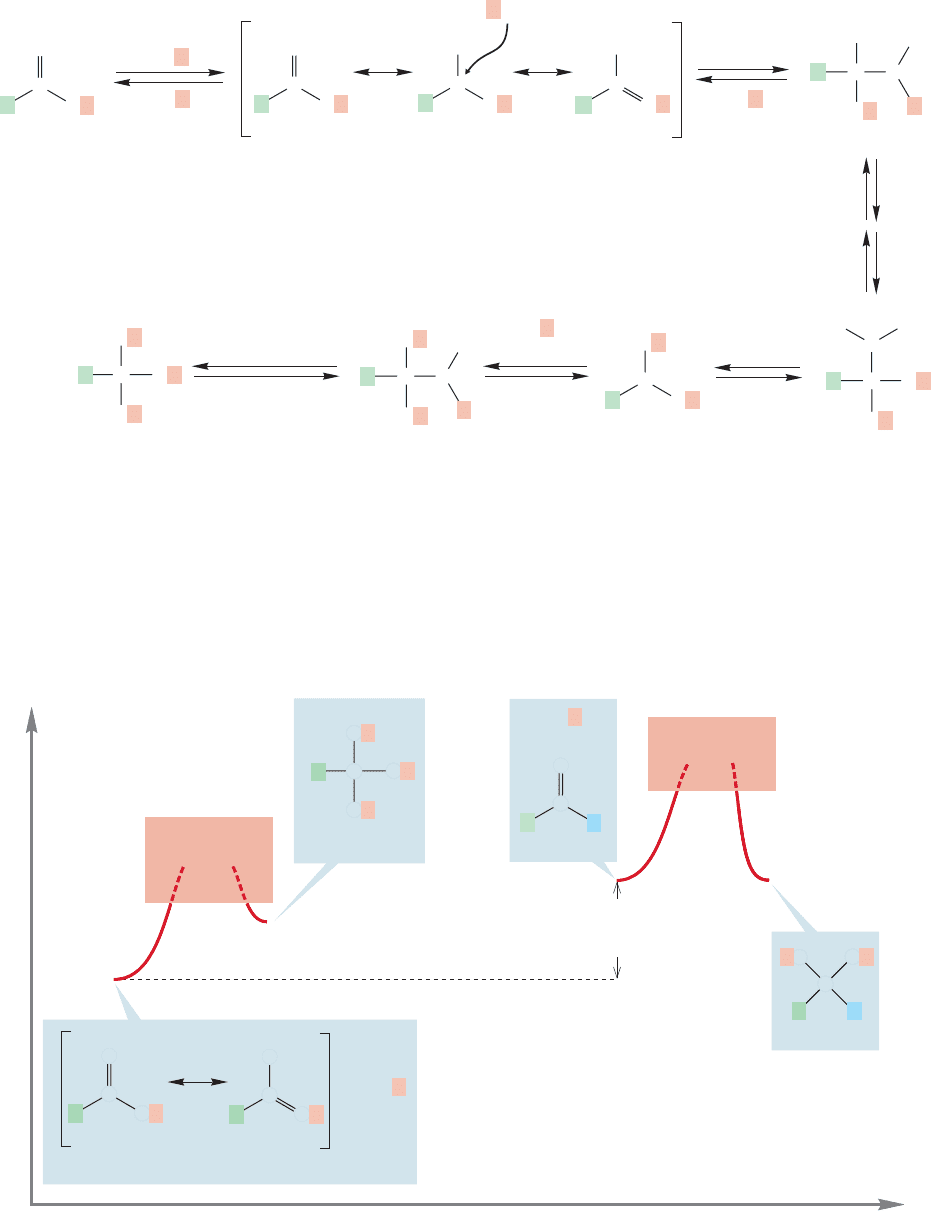
The problem is one of thermodynamics.The sequence of Figure 17.28 is a series of equi-
libria, and at equilibrium it is the ester that is greatly favored, not the ortho ester. Note
that the starting material in this reaction, the ester, is stabilized by resonance but the
product, the ortho ester, is not. The ortho ester is analogous to the thermodynamically
unstable intermediate A in Figure 17.24.Aldehydes and ketones lack this ester resonance,
and are therefore less stable, and more prone to further addition reactions. Figure 17.29
A reasonable mechanism for the conversion of an ester into an ortho ester is out-
lined in Figure 17.28. Ortho esters are known compounds, and there is nothing funda-
mentally wrong with the steps outlined in Figure 17.28. The steps are quite analogous
Energy
Energy lowering
because of
ester resonance
C
..
..
R
..
..
R
OR
RO
HOR
+
C
O
R
R
An ortho ester
Ester resonance
Reaction progress
C
..
R
OR
..
..
OR
..
..
OR
..
Acetal
HOR
+
+
–
C
O
R
OR
C
O
R
OR
..
..
..
..
..
..
..
..
..
..
..
..
..
..
Several
intermediates
Several
intermediates
Ketone
FIGURE 17.29 A truncated
Energy versus Reaction
progress diagram comparing
acetal formation from a
ketone with ortho ester
formation from an ester.
848 CHAPTER 17 Carboxylic Acids
makes this point with a partial Energy versus Reaction progress diagram.The acetal
and the ortho ester are roughly equivalent in energy, but the ester lies well below
the ketone.The result is that acetals are thermodynamically accessible from ketones,
but ortho esters are disfavored relative to esters.
There are other mechanisms for ester formation, and some of them do not involve
breaking a carbon–oxygen bond in the starting carboxylic acid. Sometimes the car-
boxylate anion is nucleophilic enough to act as the displacing agent in an S
N
2 reac-
tion.The partner in the displacement reaction must be especially reactive.In practice,
this means that a primary halide or even more reactive species such as a methyl halide
must be used (Fig. 17.30).
..
..
..
O
..
..
O
R
I
..
..
..
..
O
O
..
..
O
R
S
N
2
..
..
..
..
..
..
O
–
–
–
R
..
..
..
I
..
..
..
I
dimethyl sulfoxide
24 ⬚C
(91%)
R
..
..
O
..
..
..
O
–
..
..
..
O
..
..
..
..
OCH
3
CH
3
O
..
..
O
H
3
C
+
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 17.30 Carboxylate
anions can be used to
displace very reactive halides
in an ester-forming S
N
2
reaction.
Another example of a reaction in which the carboxylate acts as a
nucleophile is the ester-forming reaction of acids with diazomethane,
CH
2
N
2
. Diazomethane, though easily made, is quite toxic and a powerful
explosive. So, this method is generally used only when small amounts of
the methyl esters are needed (Fig. 17.31). Don’t try this reaction at home!
The first step in this reaction is deprotonation of the acid by the basic
carbon of the diazo compound (Fig. 17.32). The result is a carboxylate
anion and a diazonium ion, an extraordinarily reactive alkylating agent.
Next, an alkylation step similar to that in Figure 17.30 occurs between
the carboxylate and the diazonium ion. This reaction takes place
because nitrogen (N
2
) is a superb leaving group, perhaps the world’s best.
O
R
..
..
..
..
..
O
+
–
C CH
3
N
2
C
..
..
O
R
CH
3
..
..
O
N
N
S
N
2
..
Methyl ester
..
..
O
R
..
..
..
O
–
–
+
–
C
C
..
..
..
O
R
C
H
2
C
..
..
..
O
R
..
..
O
..
..
O
H
N
N
..
+
CH
2
N
A diazonium ionDiazomethane
Carboxylic
acid
N
..
+
++
H
FIGURE 17.32 In the first step of this methyl ester–forming reaction, diazomethane acts as a Brønsted
base and deprotonates the carboxylic acid. In the second step, the carboxylate does an S
N
2 substitution
on the very reactive methyl diazonium species.
(100%)
+
OH
CH
2
N
2
N
2
NO
2
..
..
OCH
3
C
NO
2
..
..
O
..
..
O
..
..
C
FIGURE 17.31 Diazomethane can be used to
make methyl esters from acids in yields as high
as 100%.
