Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

17.7 Reactions of Carboxylic Acids 849
In practice, this method of esterification is restricted to the synthesis of methyl
esters because other diazo compounds are relatively unstable.
A final note about ester formation addresses the case when an alcohol and a car-
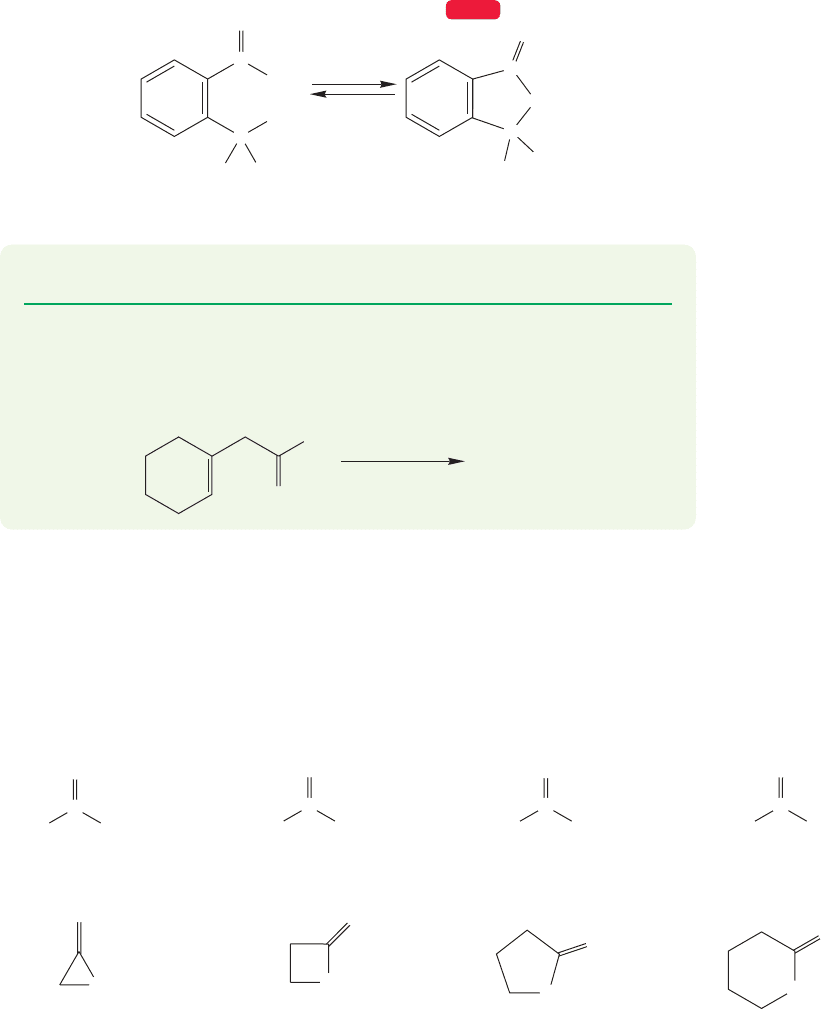
boxylic acid in the same molecule undergo esterification.The result is a cyclic ester,
known as a lactone.As usual, the relatively unstrained five- and six-membered rings
are formed more easily than other sized rings. Figure 17.33 gives an example of
lactone formation.
..
..
..
..
..
..
..
..
..
..
..
O
..
..
O
HCl
H
2
O
C
H
H
OH
OH
O
C
H
A lactone
H
..
..
C
C
WEB 3D
FIGURE 17.33 A lactone can be
formed via an intramolecular Fischer
esterification.
PROBLEM 17.14 Write a detailed mechanism for the reaction in Figure 17.33.
PROBLEM 17.15 Treatment of the compound in the figure below with I
2
/KI and
Na
2
CO
3
in water leads to a neutral molecule of the formula C
8
H
11
IO
2
. Propose
a structure for the product and a mechanism for its formation.
..
..
I
2
/KI
Na
2
CO
3
/H
2
O
C
8
H
11
IO
2
OH
..
..
O
Lactones are commonly named in reference to the acid containing the same
number of carbon atoms, with “olide” added as a suffix. Thus a butanolide has four
carbons, a pentanolide has five, and so on. A Greek letter is used to designate the
size of the ring. An α-lactone has one carbon joining the oxygen and carbonyl car-
bon,a β-lactone has two carbons,a γ-lactone three carbons, and so on.In the IUPAC
systematic naming protocol, lactones are called oxacycloalkanones (Fig. 17.34).
..
..
..
..
O
C
Ethanoic acid
(acetic acid)
OH
H
3
C
..
..
..
..
O
C
Propanoic acid
(propionic acid)
OH
CH
3
CH
2
..
..
..
..
O
C
Butanoic acid
(butyric acid)
OH
CH
3
CH
2
CH
2
..
..
..
..
O
C
Pentanoic acid
OH
CH
3
CH
2
CH
2
CH
2
Oxacyclopropanone
an ethanolide
(an α-lactone)
..
..
O
..
..
O
α
O
2-Oxacyclohexanone
a pentanolide
(a δ-lactone)
..
..
O
..
..
α
β
γ
δ
..
..
2-Oxacyclobutanone
a propanolide
(a β-lactone)
..
..
O
O
α
β
..
..
O
2-Oxacyclopentanone
a butanolide
(a γ-lactone)
O
..
α
β
γ
..
FIGURE 17.34 The naming options for lactones.
850 CHAPTER 17 Carboxylic Acids
2
As noted on p. 249, there is potential for confusion here.There are two kinds of amides, the carbonyl deriv-
ative shown here ( ) and the negatively charged ions,
NR
2
. You need to know the context
of the discussion to know which is meant. In this case we are referring to .R
O
CO
O
NHR
R
O
CO
O
NHR
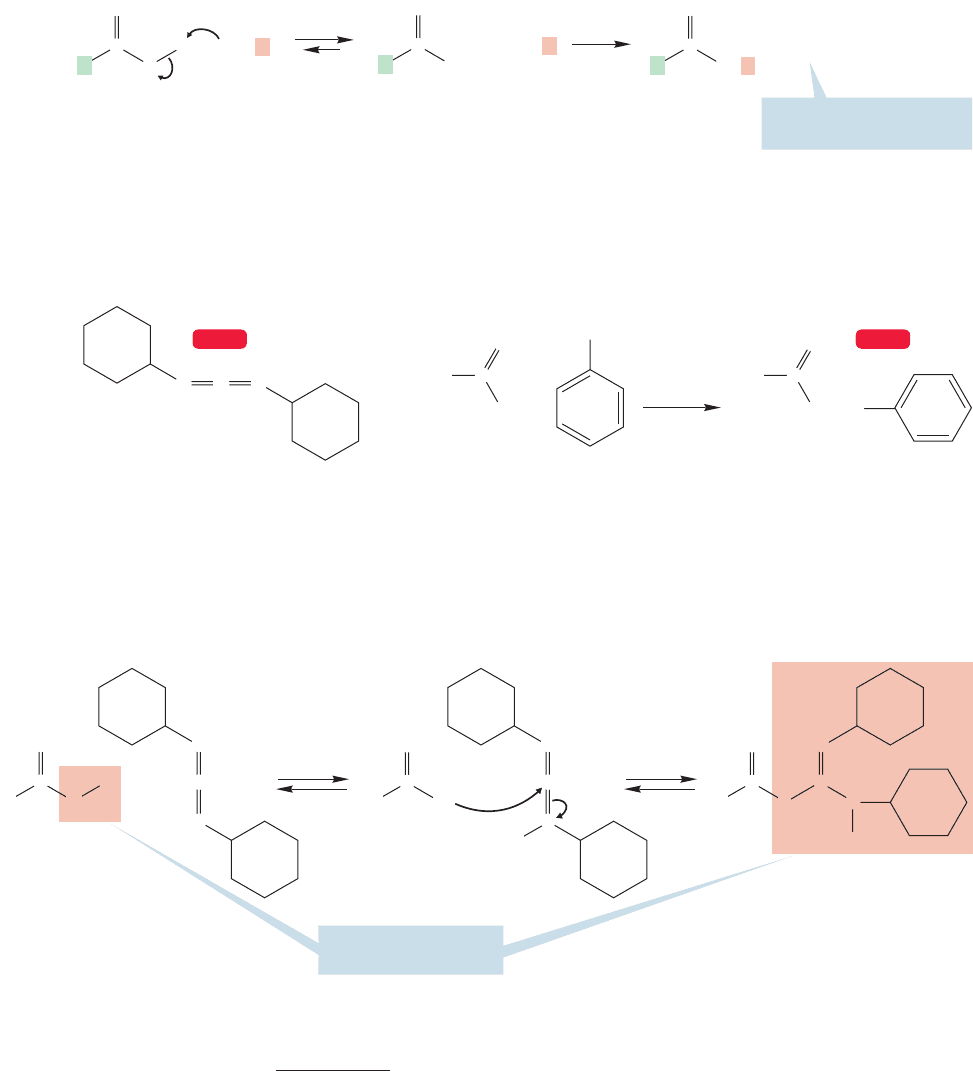
17.7b Formation of Amides Although carboxylic acids do react with primary
or secondary amines to form amides,
2
in general, this reaction is not a very useful
process. The dominant reaction between the basic amine and the carboxylic acid is
proton transfer to give the ammonium salt of the carboxylic acid. Heating of the
salts has been used as a source of amides (Fig. 17.35). However, the amount of heat
needed is more than 200 °C. Such conditions are often too harsh for the organic
compounds and result in degradation of the material.
..
..
..
O
R
..
..
O
+
–
+
C
C
..
..
..
O
R
..
..
O
H
NH
2
NH
3
R +C
..
..
..
..
..
O
R
H
2
O
R
ΔΔΔ
Acid Base An ammonium salt An amide
NHR
Notice loss of water—this
reaction is a dehydration
FIGURE 17.35 The formation of amides through the heating of ammonium
salts of carboxylic acids.
Amide formation from the carboxylic acid is much easier if the acid is first acti-
vated. Several activating agents have been developed that greatly facilitate amide
formation. Dicyclohexylcarbodiimide (DCC) is used most often (Fig. 17.36).
C
C
DCC
DCC
Amide
N
N
+
CH
2
NH
2
H
3
C
..
..
..
..
..
..
..
O
OH
C
H
3
C
..
..
..
O
NHCH
2
WEB 3DWEB 3D
FIGURE 17.36 The use of DCC, an activating agent, to produce amides.
N
–
+
A
C
..
..
O
R
H
..
O
..
..
C H
..
..
O
C
N
R
O
..
..
+
Overall change; better
leaving group formed
..
..
N
C
N
+
..
C
..
..
O
R
..
O
..
N
H
C
N
..
..
proton
transfer
FIGURE 17.37 The first step in the DCC mechanism is proton transfer.This reaction is followed by addition of the carboxylate
to one of the carbon–nitrogen double bonds. The transformation involves a change of leaving group from OH to OR to give
the activated acid A.
The strategy is to convert the poor leaving group,OH, into a better one.We used
this technique extensively in Chapter 7 (p. 282), when we discussed the transfor-
mation of alcohols. Here, the carboxylate adds to one carbon–nitrogen double bond
of DCC, accomplishing the transformation of the leaving group (Fig. 17.37).
17.7 Reactions of Carboxylic Acids 851
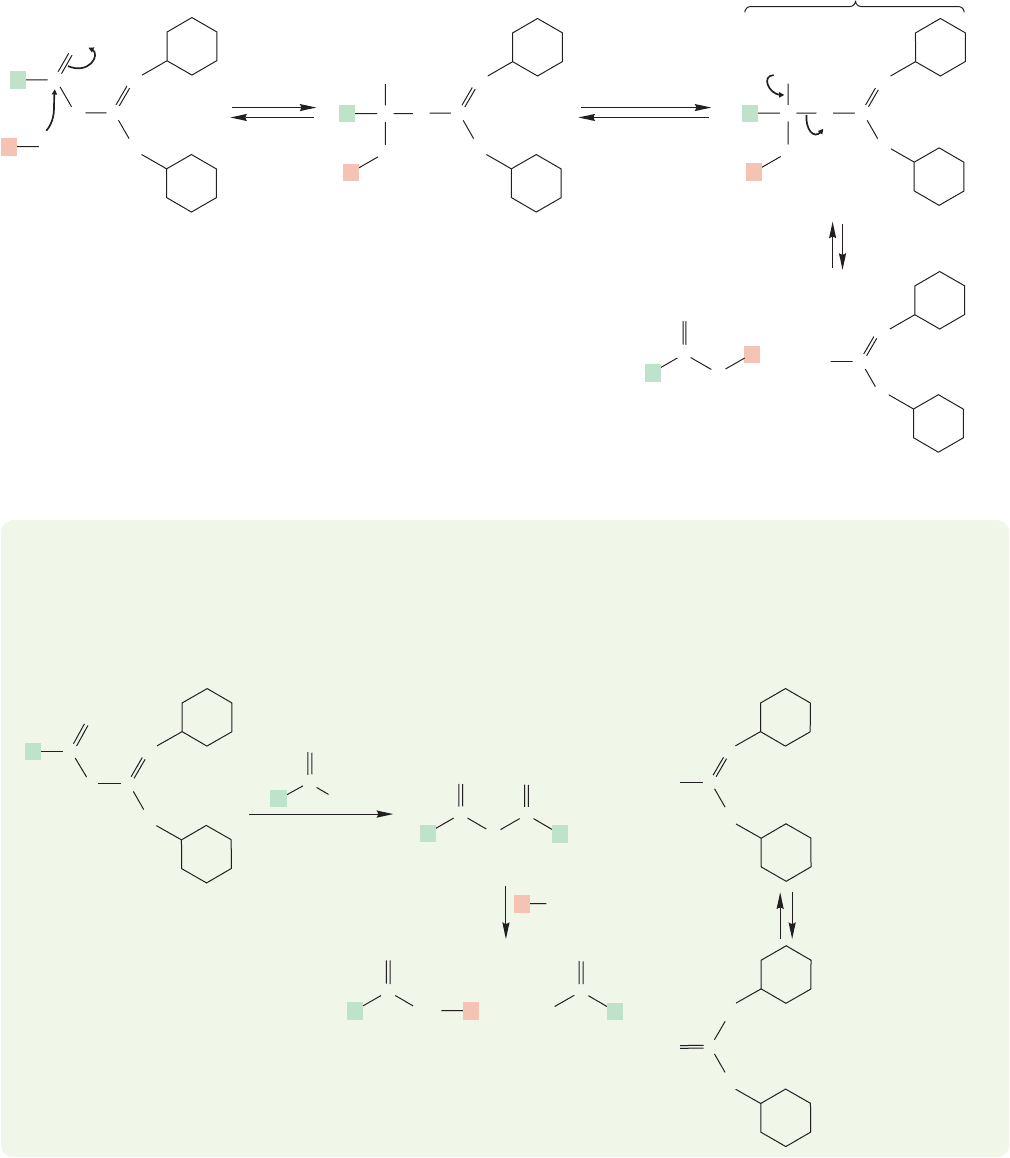
Two mechanistic pathways are now possible. In the simpler one, the primary or
secondary amine adds to the carbon–oxygen double bond to give a tetrahedral inter-
mediate that expels a relatively stable ion to generate the amide (Fig. 17.38).
If the amide is made from an amine and a carboxylic acid in the same molecule,
then a cyclic amide is formed. Cyclic amides are called lactams.
addition
step
+
–
–
deprotonation
Good
leaving group
elimination step
Amide
O
C
NH
2
O
..
..
..
..
..
C
NH
..
N
..
C
NH
..
N
..
C
N
H
..
N
..
R
R
O
C
NH
2
O
..
..
..
..
..
R
R
O
C
NH
O
..
..
..
..
..
..
R
R
Stable ion
–
C
N
H
..
N
..
..
O
..
..
R
O
C
N
H
..
..
..
R
+
A
FIGURE 17.38 Addition of the amine to intermediate A leads
to a tetrahedral intermediate that can decompose to give the
amide. Can you see why the leaving group is so good in this
reaction?
PROBLEM 17.16 There is another, more complicated (and accepted), mechanism for
the reaction of the activated acid A. The initially formed activated acid reacts not
with the amine,as in Figure 17.38, but with another molecule of carboxylic acid.The
result is an anhydride.The anhydride is the actual reagent that reacts with the amine
to give the product amide. Sketch mechanisms for anhydride and amide formation.
O
C
..
..
An anhydride
A
protonate
Amide
O
C
O
..
..
..
..
C
NH
..
N
..
NH
..
NH
..
R
O
C
O
..
..
O
..
..
..
..
..
..
R
O
C
..
..
O
C
..
..
R
R
R
C
OH
R
O
..
..
NH
2
..
..
..
NH
..
R
R
HO
C
+
C
NH
..
N
..
..
O
..
..
–
+
Summary
You have learned several methods to make carboxylic acids. We have also dis-
cussed how to make esters and amides from organic acids. Notice the similar-
ity between amide formation and ester formation. Amide formation is in fact
merely a collection of steps closely resembling those in Fischer esterification.
We are beginning to see the generality of the addition–elimination process.The
addition–elimination reaction will continue to be prominent throughout this
and other chapters.
852 CHAPTER 17 Carboxylic Acids
Nylon 6,6
OH
HO
H
2
N
O
–
O
O
–
O
O
O O O
Δ
O
O
–
–
O
O
O
O
NH
3
+
H
3
N
+
+
+
N N O
N
H
(CH
2
)
6
(CH
2
)
6
NH
2
H
2
N
(CH
2
)
6
(CH
2
)
4
(CH
2
)
4
NH
2
H
3
N
NH
3
HO
O
OH
O
(CH
2
)
4
Adipic acid Hexamethylenediamine
N
HH H
FIGURE 17.39 Formation of the polyamide nylon 6,6.The compound in the third line is shown truncated, which is
what the squiggly line through the bond means.
17.7c Polyamides (Nylon) and Polyesters The reaction shown at the
start of Section 17.7b, the heating of the ammonium salt of a carboxylic acid to
give an amide, is at the heart of the synthesis of a set of polyamides collectively
known as nylons. This reaction is certainly not insignificant, as evidenced by the
fact that the annual worldwide production of nylon is 4 million tons.
3
In one
example of nylon synthesis, a double-headed amine, hexamethylenediamine,
reacts with a double-headed acid, adipic acid, to form a salt (Fig. 17.39).
Pyrolysis (extreme heating) of this salt at 275 °C produces the long-chain poly-
mer known as nylon 6,6. Nylons of other chain lengths are also produced by the
chemical industry.
3
This information is obtained from Yarns and Fibers Exchange (YNFX), 2007.
Polyesters are similarly made through the reactions of diacids with diols—simple
Fischer esterification reactions that produce a polymeric structure.For example,Dacron
and Mylar are trade names for different polyesters that are made from ethylene glycol
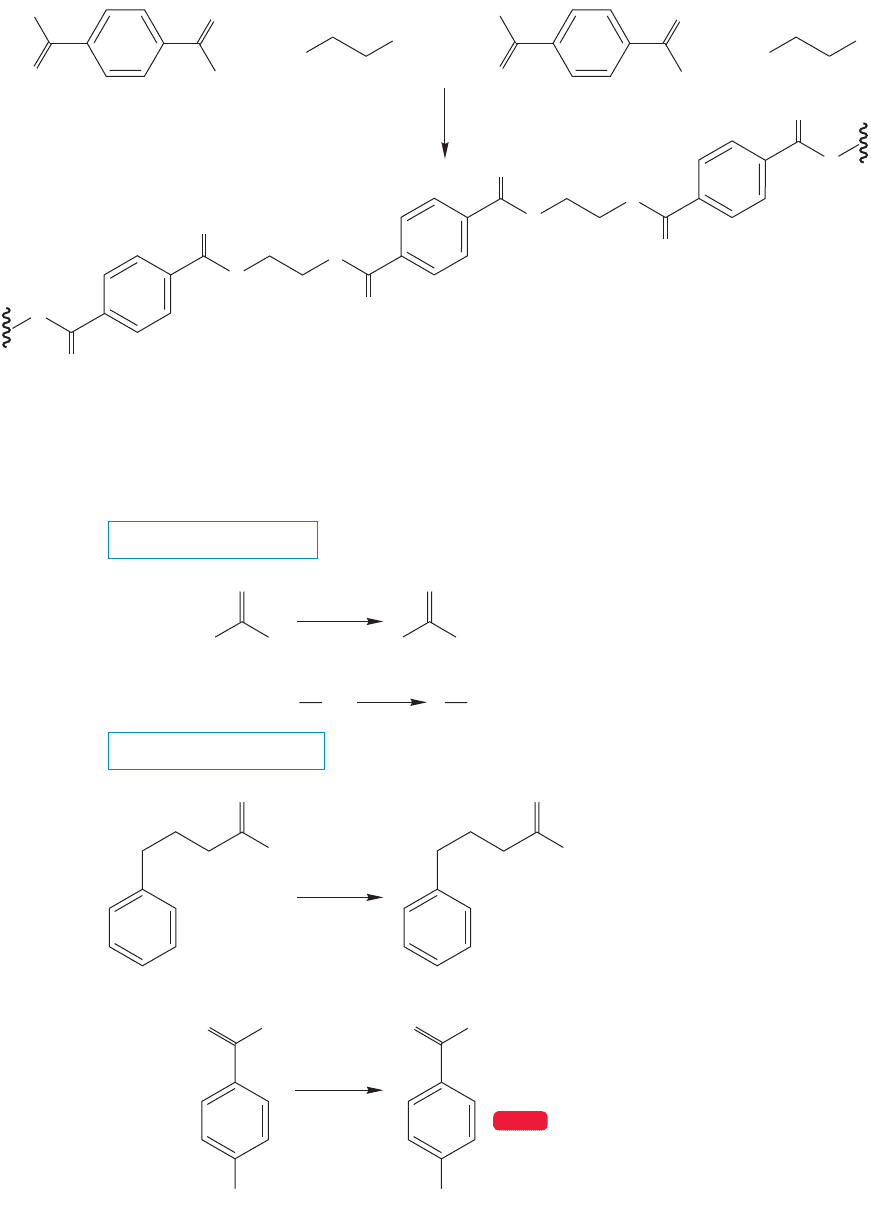
and terephthalic acid (Fig. 17.40). This type of polyester, polyethylene terephthalate
(PET), is also used to make clear recyclable plastic bottles. It is more recyclable and
therefore more environmentally friendly than most other kinds of polymers.
17.7 Reactions of Carboxylic Acids 853
HO
Fischer esterificationTerephthalic acid
OH
HO
OH
HO
HO
O
HO
O
O
HO
O
O
O
O
O
O
O
O
O
O
O
O
O
FIGURE 17.40 Polyester synthesis.
This particular polyester is used in
many consumer products.
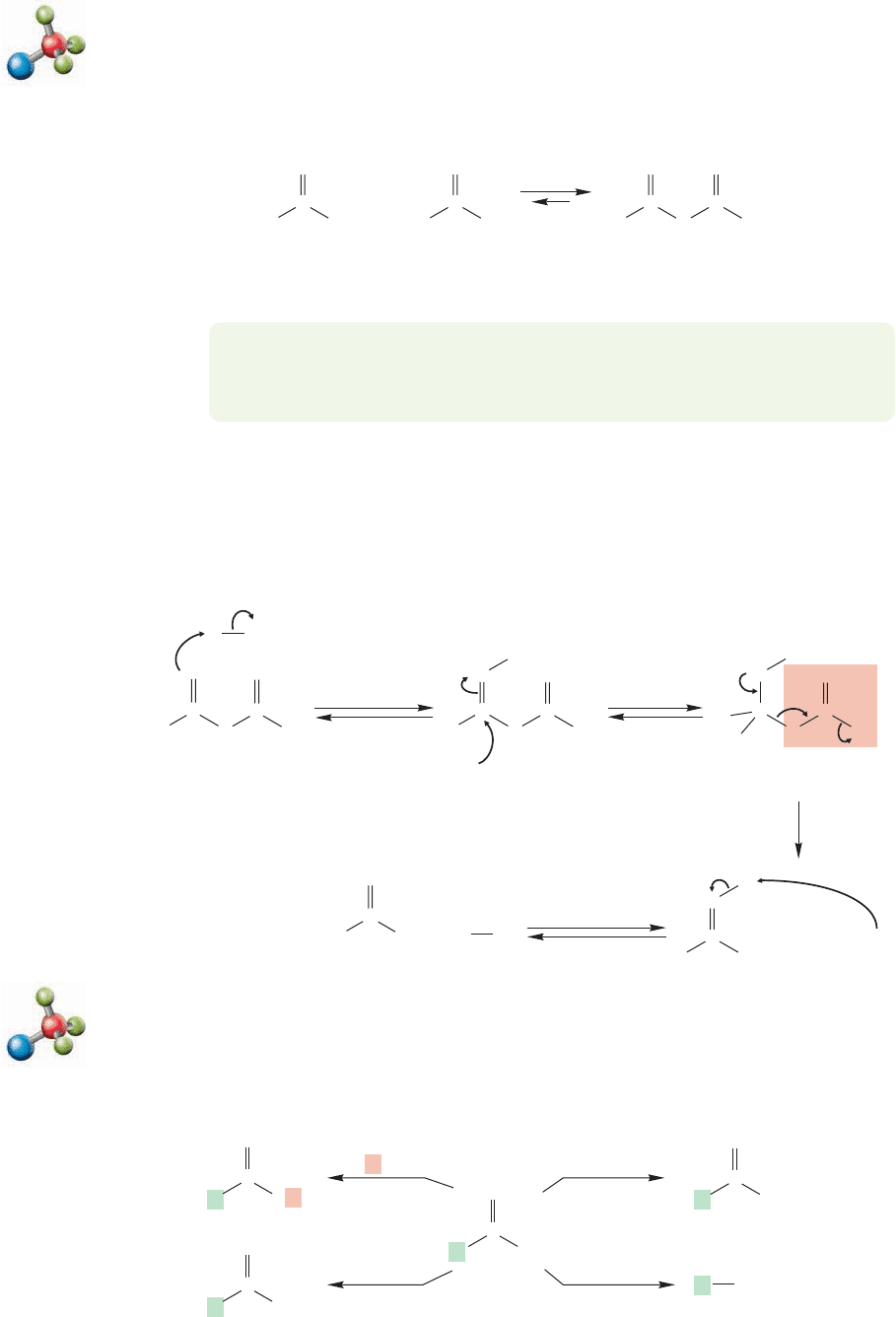
17.7d Formation of Acid Chlorides We saw acid chlorides in our study of
Friedel–Crafts acylation (p. 643). One can make an acid chloride in good yield by
treatment of a carboxylic acid with thionyl chloride or phosphorus pentachloride
(Fig. 17.41).This reaction closely resembles the formation of chlorides by the reaction
O
OH
SOCl
2
100 ⬚C
(>90%)
..
..
..
..
O
Cl
..
..
..
..
..
O
OH
PCl
5
NO
2
100 ⬚C
(93%)
..
..
..
..
O
Cl
NO
2
..
..
..
..
..
SOCl
2
Recall from Chapter 7: R OH R Cl
..
..
..
..
..
SPECIFIC EXAMPLES
WEB 3D
O
OHR
SOCl
2
..
..
..
..
O
ClR
..
..
..
..
..
THE GENERAL CASE
FIGURE 17.41 The reaction of
carboxylic acids with thionyl chloride
(SOCl
2
) or phosphorus pentachloride
(PCl
5
) gives acid chlorides. The
reaction of alcohols with SOCl
2
is similar.
854 CHAPTER 17 Carboxylic Acids
of alcohols with thionyl chloride (p. 284). Once again, the key concept in acid
chloride formation is activation of the carboxylic acid by the transformation of a
very poor leaving group, OH, into a better one. In the first step of the acid chloride
formation using SOCl
2
, a chloride is displaced to form an activated acid interme-
diate, in this case a chlorosulfinic acid derivative (Fig. 17.42).
O
..
..
O
C
..
..
O
..
..
R
O
C
..
..
R
S
..
..
+
..
..
OH
..
..
.. ..
Cl
..
..
Cl
O
..
..
S
..
..
Cl
..
..
..
..
HCl
The activated acid,
a chlorosulfite ester
Thionyl chlorideCarboxylic
acid
+
FIGURE 17.42 An activated acid is
formed in the first step of acid
chloride formation.
O
S
..
..
O
C
O
..
..
..
..
.. .. ..
R
..
..
..
Cl
H
H
O
S
..
..
O
C
O
..
..
..
R
..
..
..
Cl
..
..
..
..
Cl
H
+
–
_
SO
2
O
C
Cl
..
..
R
..
..
..
..
Cl
H
+
++
+
protonation
Activated acid
Acid chloride
addition
deprotonation
O
S
..
..
O
C
O
..
..
..
..
R
..
..
..
Cl
..
..
..
Cl
H
elimination
Good leaving
group
..
..
O
C
Cl
..
..
R
..
..
..
Cl
..
..
..
..
..
..
Cl
FIGURE 17.43 Hydrogen
chloride protonates the
carbonyl group, and
chloride attacks this strong
electrophile. In the crucial
step, sulfur dioxide and
chloride ion are lost as the
acid chloride is formed.
Esters
A
mides
Carboxylic
acids
AlcoholsAcid
chloride
C
R
R
Cl
R
R
R R
R
..
..
..
..
..
..
..
O
..
..
O
O
..
..
NH
2
O
..
..
..
..
OH
O
..
..
..
..
..
..
CH
2
OH
..
..
OH
NH
3
2. H
2
O
..
..
H
2
O
1. LiAlH
4
C
C
C
C
FIGURE 17.44 Some
compounds formed from
addition–elimination
reactions between
different nucleophiles
and acid chlorides. See
Problem 17.18.
PROBLEM 17.17 Write a mechanism for the formation of the product in Figure
17.42. Watch out for the details! Almost everyone makes a small error the first
time on this problem.
The hydrogen chloride generated in the formation of the activated acid proto-
nates the carbonyl oxygen, and the chloride ion adds to the strong electrophile.The
tetrahedral intermediate then breaks down into chloride, sulfur dioxide,and the acid
chloride. Notice the change in leaving group induced by the overall transformation
of OH into OSOCl (Fig. 17.43).
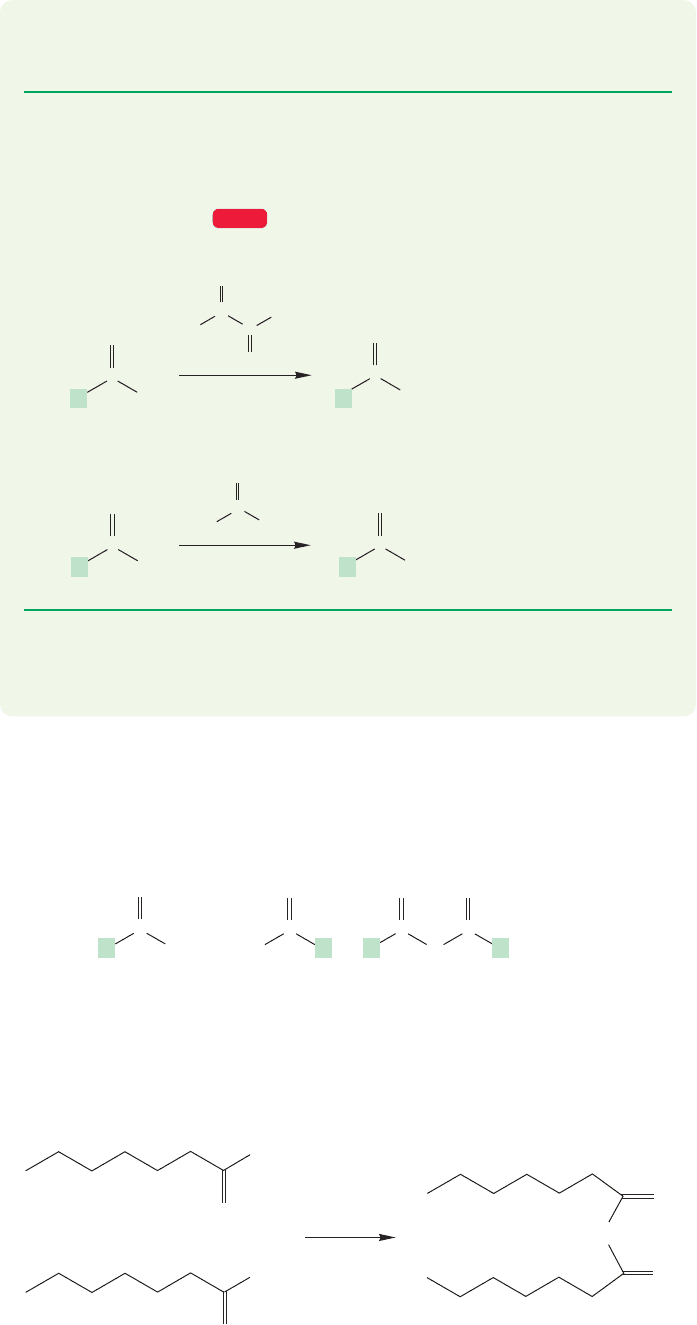
Acid chlorides contain a good potential leaving group, the chloride; and the
addition–elimination process can lead to all manner of acyl derivatives, ,
as we will see in Chapter 18 (Fig. 17.44).
R
O
CO
O
X
Acid chloride formation
Acid chloride aminolysis
17.7 Reactions of Carboxylic Acids 855
PROBLEM 17.18 Write a mechanism for the transformation of an acid chloride
into a primary alcohol as shown in Figure 17.44.
PROBLEM 17.19 Another excellent synthesis of acid chlorides from carboxylic acids
uses either phosgene ( ) or oxalyl chloride ( )
as the reactive agent. Suggest a mechanism for the reaction with phosgene.
Cl
O
CO
O
CO
O
ClCl
O
CO
O
Cl
Oxalyl chloride
Phosgene
Cl
Cl
C
C
OH
..
O
..
..
..
..
..
+
O
..
..
..
CO
2
+ CO + HCl
+ CO
2
+ HCl
Cl
..
..
..
C
O
..
..
Cl
..
..
..
Cl
..
..
..
C
Cl
..
..
..
C
OH
..
O
..
..
..
R R
R R
O
..
..
O
..
..
O
..
..
C
C
WEB 3D
PROBLEM 17.20 Phosgene (see Problem 17.19) is a most effective poison.Can you
guess its mode of action? What happens when phosgene is absorbed by moist
lung tissue?
Two carboxylic
acids
Anhydride Water
.. ..
O
..
..
O
..
..
..
.. ..
OH
O
HO
..
..
H
2
O
..
O
O
..
..
..
..
RR R
R
+=C
C
C
C
FIGURE 17.45 A comparison
between two carboxylic acids and
acid anhydrides.
17.7e Anhydride Formation Acid anhydrides are, as the name suggests,
related to carboxylic acids by a formal loss of water. An anhydride is “two carboxylic
acids less a molecule of water” (Fig. 17.45).
+
(80%)
O
60 ⬚C
benzene
..
..
O
..
..
O
..
..
O
..
..
–
O
..
..
..
O
..
..
..
..
..
Cl
FIGURE 17.46 Anhydride formation
through the reaction of a carboxylate
salt with an acid chloride.
Carboxylic acids and their conjugate bases, the carboxylate anions, react with acid
halides to give acid anhydrides (Fig. 17.46).
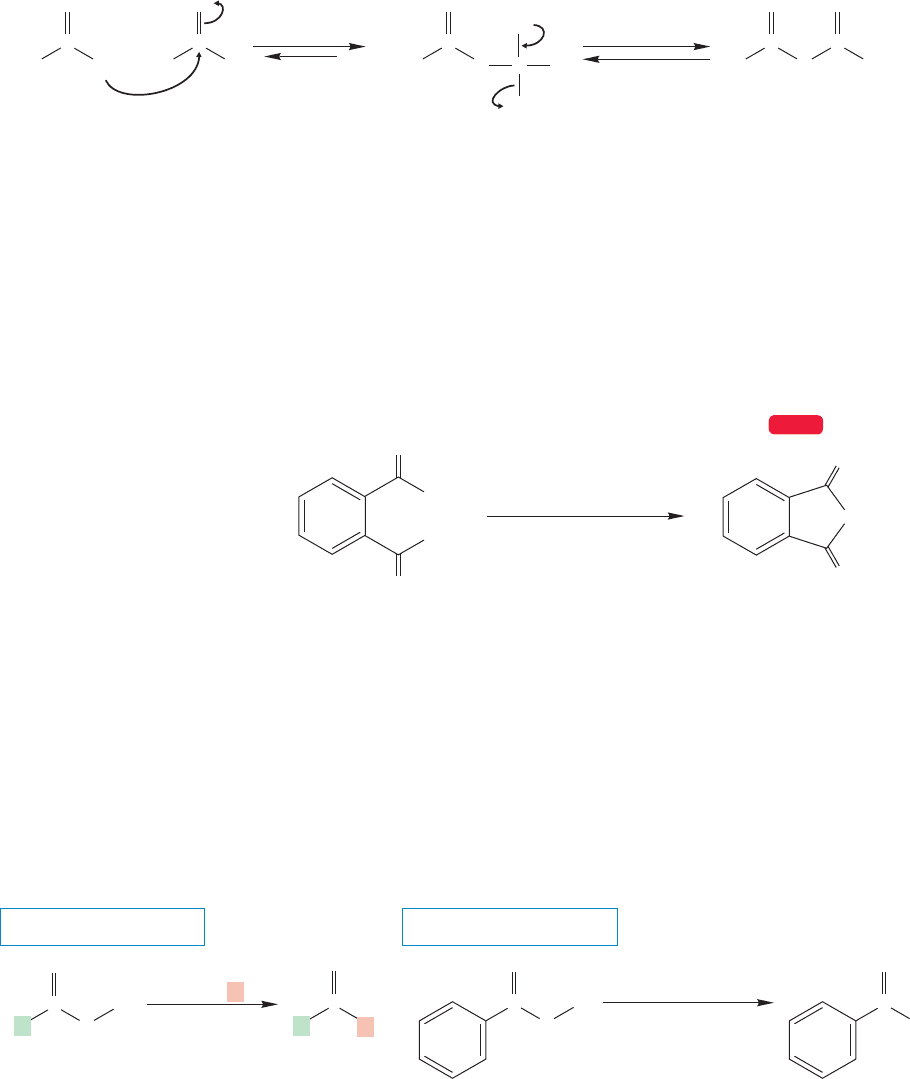
–
addition elimination
Tetrahedral
intermediate
Anhydride
..
..
..
O
O
..
..
O
..
..
..
+
..
Cl
..
..
–
C
O
..
..
..
..
Cl
..
R
..
..
O
O
..
..
R
..
..
O
O
..
..
O
..
..
RRR
R
..
..
+
Cl
..
..
–
CC C CC
FIGURE 17.47 The mechanism of anhydride formation involves the generation of a tetrahedral intermediate and
loss of chloride ion.
Phthalic acid
melt
Phthalic anhydride
200 ⬚C
O
..
..
O
..
..
H
2
O
O
..
..
O
..
..
..
..
+
H
..
..
O
H
..
..
..
O
O
..
WEB 3D
FIGURE 17.48 Dehydration of a dioic acid can form a cyclic anhydride.
O
H
1. 2 equiv. Li
O
..
..
..
C
O
..
..
..
..
O
H
O
..
..
O
..
..
..
..
..
1. 2 equiv. (CH
3
)
3
CLi
pentane, 25 °C
2. H
2
O
..
..
2. H
2
O
C(CH
3
)
3
(67%)
R
R
RR
THE GENERAL CASE
A SPECIFIC EXAMPLE
C
CC
FIGURE 17.49 A general ketone synthesis from a carboxylic acid and an organolithium reagent. Note again
the convention that differentiates sequential reactions (written 1. RLi; 2. H
2
O) from “dump ’em all together”
reactions (written RLi, H
2
O).
856 CHAPTER 17 Carboxylic Acids
The mechanisms for these reactions involve addition of the carboxylate anion to
the carbonyl group of the acid chloride to give a tetrahedral intermediate that can
lose a chloride ion, which is a good leaving group (Fig. 17.47).
Other reagents such as DCC or P
2
O
5
can be used to form anhydrides. Such
reagents are called dehydrating reagents. Cyclic anhydrides can be formed by pyrol-
ysis, or thermal dehydration, of the diacid (Fig. 17.48).
17.7f Reactions with Organolithium Reagents and Metal Hydrides
In a useful synthetic reaction that was mentioned on p. 837, organic acids react
with two equivalents of an organolithium reagent to give the corresponding ketone
(Fig. 17.49).
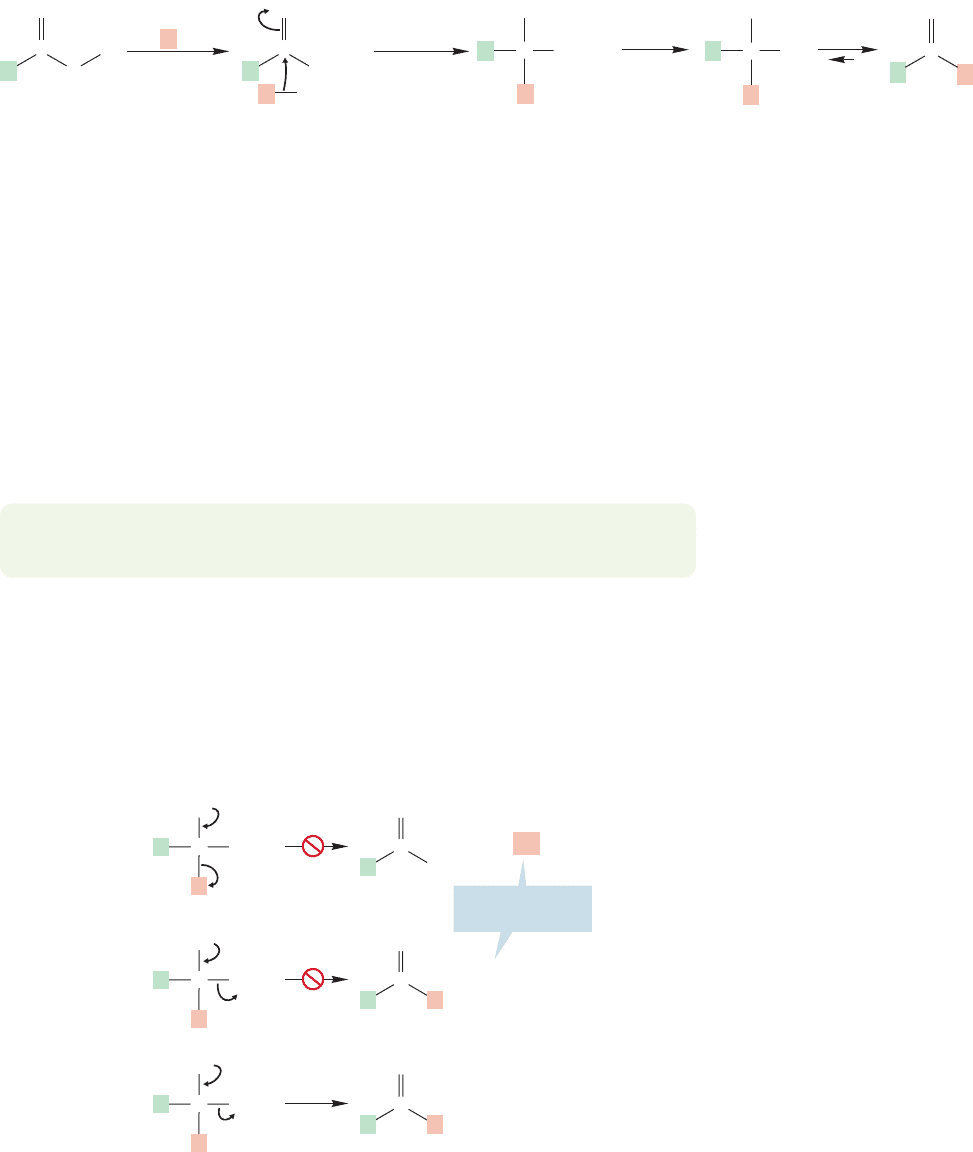
The first step in the reaction is the formation of the lithium salt of the carboxylic
acid.Although this species already bears a negative charge,the organolithium reagent
is a strong enough nucleophile to add to it to give the “dianion” (Fig. 17.50).
17.7 Reactions of Carboxylic Acids 857
deprotonation
protonation
addition
Dianion
C
O
H
Li
Li
O
1
..
..
..
..
+
–
–
..
..
..
..
–
O
OC
..
..
..
Li
+
Li
+
R
Li
R
R
R
C
O
O
1
..
..
..
..
R
R
Hydrate
Ketone
C
R
R
..
..
OH
OH
H
3
O
H
2
O
..
C
+
R
R
..
..
..
..
O
..
..
FIGURE 17.50 The first step in the reaction is formation of the carboxylate anion. In a second step, the organolithium
reagent is a strong enough nucleophile to add to the carboxylate anion to give a lithium-stabilized dianion. When
water is added to the reaction, a hydrate is formed. Hydrates are generally unstable compared to ketones.The
equilibrium favors the final product of the reaction, the ketone.
What can happen to the product dianion in this basic solution? This question
is one of the rare instances when the answer really is, “nothing.”There is no possi-
ble leaving group, because neither R
nor O
2
can be lost. So, the dianion remains
in solution until water is added to the reaction mixture, at which point a hydrate is
formed. Hydrates are unstable relative to their ketone precursors (p.777), and so the
end result is the ketone.
O
C
+
Li
+
Li
+
Li
+
–
–
C
O
O
–
–
..
O
R
R
R
R
Leaving groups?
No!
This reaction is fine, as long as L is a good leaving group
O
C
+
Li
+
Li
+
Li
+
–
–
–
C
O
O
2–
..
R
R
R
R
O
C
+
R R
–
C
L
L
O
R
R
O
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
FIGURE 17.51 The dianion from
Figure 17.50 is foiled in any attempt
to expel a negative charge because
of the absence of a possible
leaving group.
PROBLEM 17.21 What reagent(s) would you use to synthesize acetophenone from
acetic acid?
The key to the formation of the ketone is the inability of the dianionic inter-
mediate to expel a leaving group. Compare this nonreaction with a general process
in which a leaving group is present (Fig. 17.51). We have already seen many reac-
tions of this type, and in Chapter 18 we will see many more.
858 CHAPTER 17 Carboxylic Acids
C
1. LiAlH
4
2. H
2
O
OHR
RCH
2
(97%)
1. excess
LiAlH
4
2. H
2
O
HOOC COOH(CH
2
)
8
HOCH
2
CH
2
OH(CH
2
)
8
OH
..
..
..
..
..
..
O
..
..
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 17.52 The reduction of carboxylic acids to alcohols.
O
C
LiAlH
4
+ H
2
+ AlH
3
+
+
Tetrahedral
intermediate
Carboxylic
acid
O
H
R
..
..
O
C
R
C
O
R
LiAlH
4
Aldehyde
(cannot survive
in LiAlH
4
)
–
Li
+
Li
+
Li
+
–
O
O
–
OAlH
2
R
H
AlH
2
H
..
..
..
..
..
..
..
O
..
..
..
..
..
O
..
..
C
LiAlH
4
C
R
H
H
2
O
H
..
..
OAlH
2
..
..
..
..
..
C
R
H
H
HO
–
..
..
..
OH
..
..
FIGURE 17.53 Deprotonation to give the carboxylate anion is followed by hydride
addition to give a “dianion” bound to aluminum. Loss of LiOAlH
2
leads to an
intermediate aldehyde, which is reduced by a hydride. At the conclusion of the reaction,
protonation by water gives the alcohol.
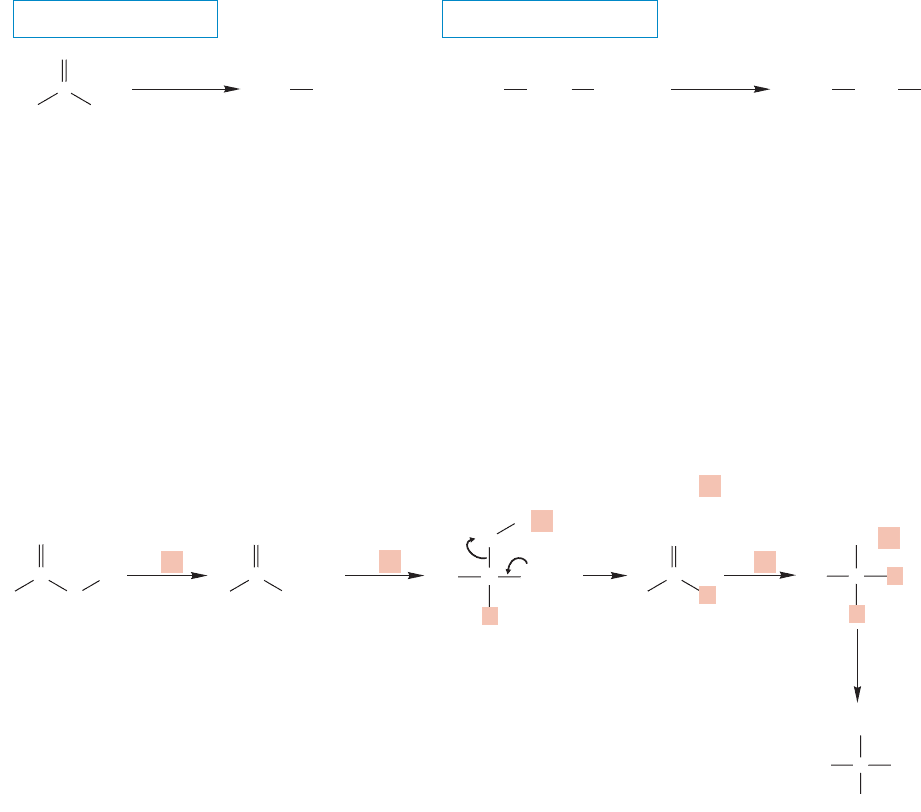
17.7g Decarboxylation Some carboxylic acids easily lose carbon dioxide in a
reaction logically called decarboxylation. The best examples are β-keto acids, and
1,3-diacids such as malonic acid (propanedioic acid) in which there is also a car-
bonyl group β to the acid (Fig. 17.54). The reactions often take place at room tem-
perature or on gentle heating. Note that the decarboxylation reaction of a β-keto
acid provides a new route for syntheses of a ketone and the decarboxylation of a
1,3-diacid is a new route for formation of carboxylic acids.
Reduction of carboxylic acids by lithium aluminum hydride, followed by
hydrolysis, gives the corresponding primary alcohols (Fig. 17.52). There is no
unanimity on the details of the reaction mechanism, but the first step must
be formation of the carboxylate anion and hydrogen. Anion formation is followed
by addition of hydride to the carbonyl group. The result is another “dianion.” In
this case, the aluminum is undoubtedly tightly bound to one of the oxygen anions.
Now, unlike the “dianion” in Figure 17.50, a molecule of aluminum oxide can be
lost to generate the aldehyde (Fig. 17.53). Because the LiAlH
4
can continue to
provide hydride (some experiments show 3.8 equivalents of hydride come from
LiAlH
4
), the newly formed aldehyde cannot survive for long (p. 801). Another
addition of hydride gives the alkoxide.The alkoxide is stable in the basic environ-
ment and subsequent addition of water forms the primary alcohol.
