Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

17.7 Reactions of Carboxylic Acids 859
..
Ketones
Malonic acids
Acids
Monoester of
carbonic acid
Alcohols
+
β-Keto acids
β
..
..
O
R
H
..
..
..
..
O
..
..
O
α
..
..
O
O
..
..
O
Δ
R
CO
2
+ CO
2
CO
2
+
+
CO
2
+ CO
2
+ CO
2
H
3
C
H
3
C
(> 60%)
(> 70%)
(~100%)
CH
3
..
..
OH
C
~25 ⬚
..
..
O
..
..
O
H
..
..
O
..
..
O
..
..
O
Δ
Δ
HO
CH
3
..
..
HO
..
..
..
O
..
..
O
..
..
OH
..
HO
..
C
~25 ⬚
..
..
O
..
HO
..
..
..
O
..
..
HO
..
..
OR
..
HOR
..
O
2
N
..
..
O
..
..
..
..
O
O
H
30 ⬚C
O
2
N
..
..
OH
GENERAL REACTIONS
SPECIFIC EXAMPLES
WEB 3D
FIGURE 17.54 Three kinds of carboxylic acid that decarboxylate (lose CO
2
) easily.
O
Δ
(b)
CO
2
H
Δ
(a)
CO
2
HHO
2
C
We will defer discussion of the decarboxylation of β-keto acids until we learn
the utility of such compounds in Chapter 19, but carbonates and malonic acids will
be mentioned here.
Carbonic acid (H
2
CO
3
or HOCOOH) is itself unstable, although its salts are
isolable and familiar as sodium bicarbonate and sodium carbonate:
PROBLEM 17.22 Show the product for each of the following reactions:
Carbonic acid
(unstable)
Sodium bicarbonate
(stable)
Sodium carbonate
(stable)
..
..
..
..
O
Na
O
OH
..
..
O
C
C
H
H
..
..
O
..
..
O
+
–
–
..
..
..
..
..
..
..
O
Na
O
O
C
+
Na
+
–
..
..
..
..
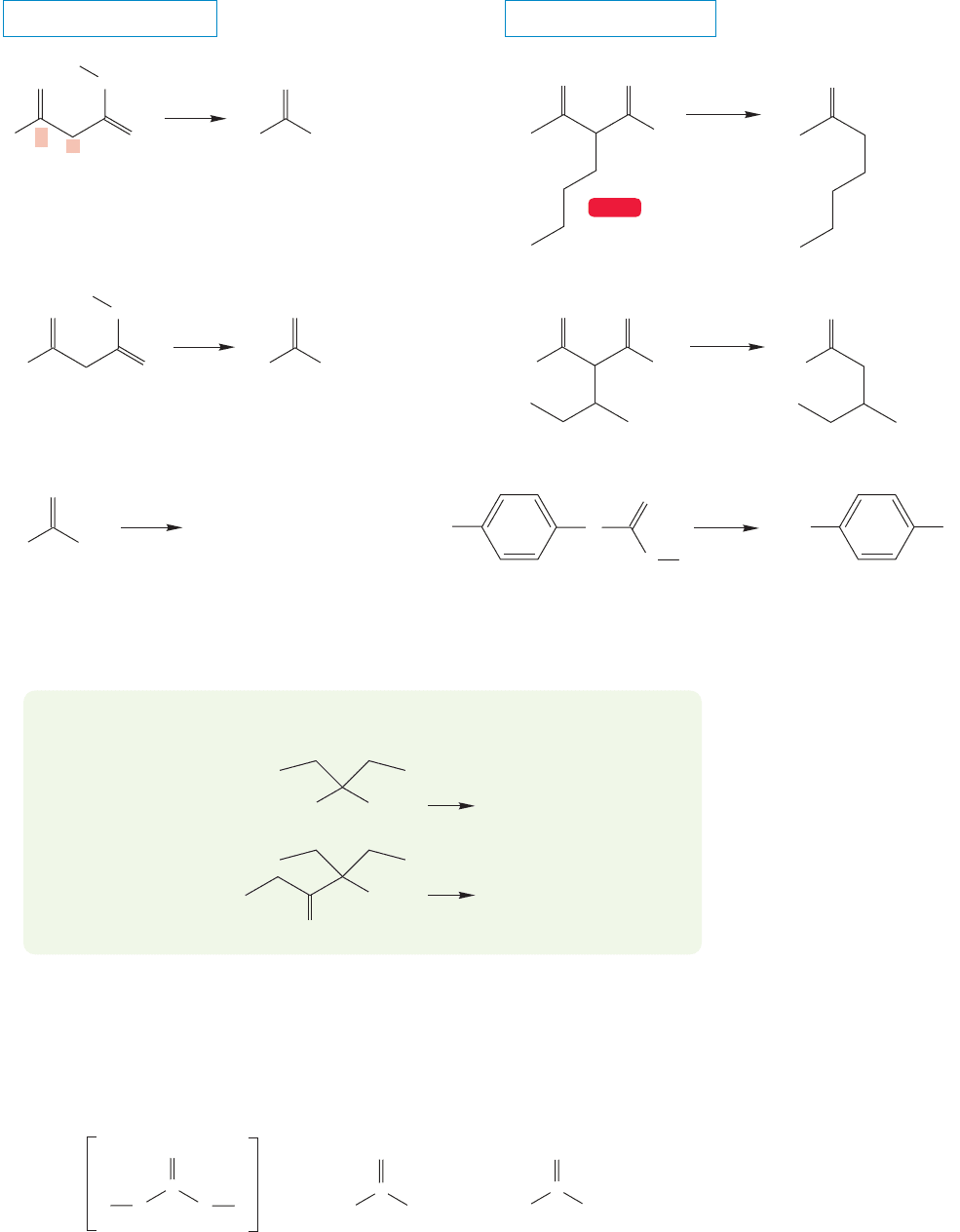
860 CHAPTER 17 Carboxylic Acids
FIGURE 17.55 Monoesters of
carbonic acid decarboxylate easily, but
carbonates (acid diesters) do not.
Monoesters of carbonic acid lose carbon dioxide easily. The loss of carbon dioxide
is irreversible, because the gaseous carbon dioxide escapes.The diesters of carbonic
acid (carbonates) are stable (Fig. 17.55).
Carboxylic acids can be electrochemically decarboxylated to give, ultimately, a
hydrocarbon composed of two R groups. This reaction, called the Kolbe electrolysis
after Hermann Kolbe (1818–1884), involves an electrochemical oxidation of the
carboxylate anion to give the carboxyl radical. Loss of carbon dioxide gives an alkyl
radical that can dimerize to give the hydrocarbon (Fig. 17.57).
Carbamic acid Urea
(stable)
Carbamate
(stable)
..
..
O
C
H
2
N H
..
NH
3
..
..
..
O
+
..
..
O
C
..
R
..
..
O
C
H
2
N
..
..
..
O
H
2
NNH
2
..
CO
2
WEB 3D
FIGURE 17.56 Carbamic acids
decarboxylate, but urea and
carbamates are stable.
electrochemical
oxidation
Kolbe
..
..
O
R
O
R
M
CO
2
..
O
+
+
R
.
O
R
2 CO
2
( 75%)
..
..
..
O
..
O
..
..
O
..
..
..
+
–
–
..
..
..
..
..
..
O
..
..
O
THE GENERAL CASE
A SPECIFIC EXAMPLE
.
R
FIGURE 17.57 The Kolbe electrolysis is a source of molecules, , made up of two R groups from .R
O
COOHR
O
R
Stable carbonate
(no H to be removed)
CO
2
++
..
..
O
C
..
..
O
..
..
O
..
..
O
C
..
..
O
..
ROH
2
..
..
..
RO
..
..
HOR
..
..
O
RR
HR
–
+
Similarly, carbamic acid, the amide derivative of carbonic acid, is also unstable,
and loses carbon dioxide to give amines (Fig. 17.56). The diamide, urea, and car-
bamates, which cannot lose carbon dioxide, are stable.
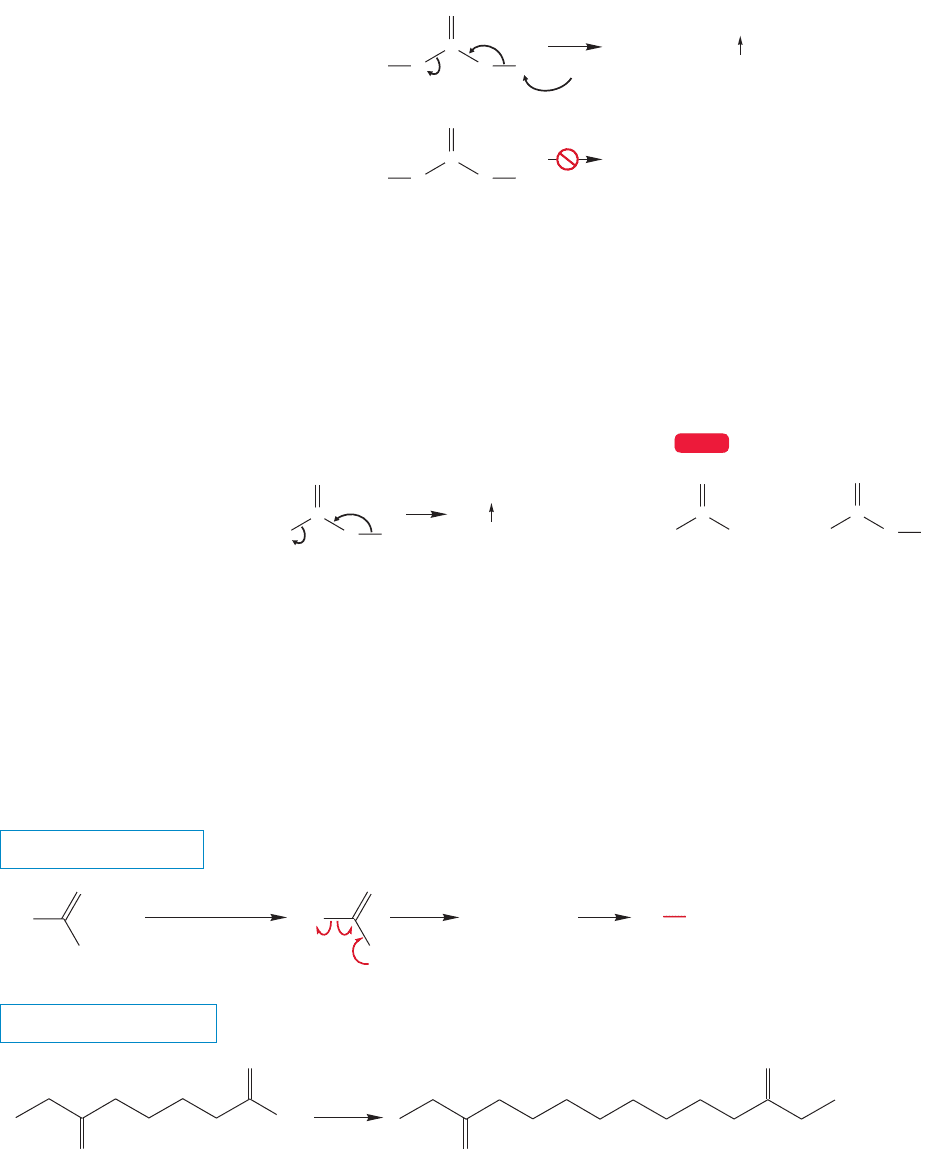
17.7 Reactions of Carboxylic Acids 861
17.7h Formation of Alkyl Bromides: The Hunsdiecker Reaction The
carboxyl radical encountered in Figure 17.57 is also the active ingredient in the
Hunsdiecker reaction in which alkyl bromides are formed from acids (Heinz
Hunsdiecker, 1904–1981; Cläre Dieckmann Hunsdiecker, 1903–1995). Although
this reaction is named for the Hunsdieckers, this reaction was actually discovered
by Alexander Borodin, the Russian composer-physician-chemist. Borodin is far
more famous for his somewhat schmaltzy music rather than his excellent chem-
istry. This reaction was first done by Borodin in 1861, 81 years before the
Hunsdieckers! Typically, the silver salt of the acid is used and allowed to react with
bromine.The first step is the formation of the hypobromite (Fig. 17.58). On heat-
ing, the weak oxygen–bromine bond breaks homolytically to give the carboxyl rad-
ical and a bromine atom. As in the Kolbe reaction, the carboxyl radical undergoes
decarboxylation to give an alkyl radical. In this case, however, the alkyl radical can
carry the chain reaction further by reacting with the hypobromite to produce an
alkyl bromide and another molecule of carboxyl radical as shown in Figure 17.58.
For a review of radical chain reactions, see Chapter 11, pp. 481ff.
R
O
O
Ag
AgBr
..
..
..
..
..
CO
2
A hypobromite
Product Recycles
..
R
O
..
..
..
..
–+
+
+
+
Br
..
..
..
Br
..
..
..
R
O
..
..
Br
..
..
..
Ag
–+
.
.
Br
..
..
..
Br
..
..
..
.
.
.
.
R
.
.
R
Br
..
..
..
O
CH
3
O
Br
2
O
O
..
..
O
..
..
..
..
..
..
..
..
..
(67%)
BrCH
3
O
..
..
CCl
4
Δ
..
..
R
O
O
..
..
..
..
Br
..
..
..
R
O
O
..
..
..
O
..
..
..
R
O
O
..
..
..
..
.
.
O
R
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 17.58 The decarboxylation
of the carboxylate radical is also
involved in the Hunsdiecker reaction,
a synthesis of alkyl bromides from
carboxylic acids. The recycling, chain-
propogating carboxyl radical is
highlighted.
Summary
That’s a lot of material. Many new kinds of compounds have appeared and there
may seem to be an overwhelming number of new reactions.Don’t be dismayed! Most
involve versions of the addition–elimination reaction. Keep this generalization
in mind and you will be able to figure out what is happening in these reactions
relatively easily. It is not necessary, or productive, to try to memorize all this
material. What is far more effective is to learn the basic process well, and then
practice applying it in different circumstances.
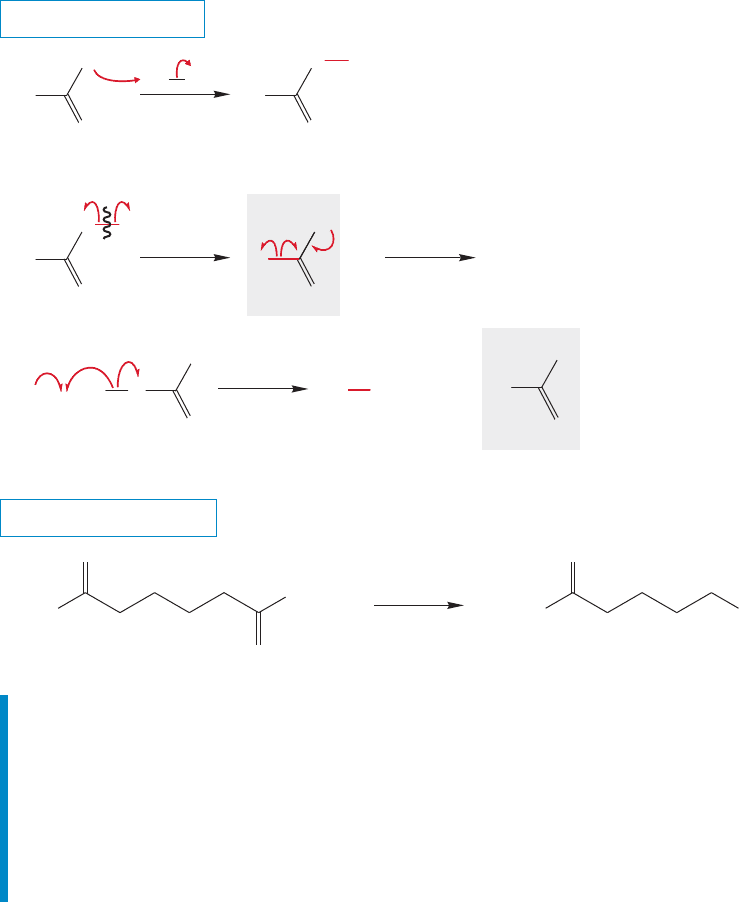
862 CHAPTER 17 Carboxylic Acids
PROBLEM 17.23 Suggest a mechanism for the hydroxide ion–induced hydrolysis
(saponification) of a fat to a fatty acid.
In this chapter we learned that carboxylic acids can be used to make
polyamides and polyesters. We also learned the reactions that use organic acids
to make acid chlorides, acid anhydrides,esters, amides, ketones, and primary alco-
hols. Finally, we’ve learned about a few decarboxylation reactions of carboxylic
acids. Perhaps the most important of the reactions in this chapter are acid chlo-
ride formation and Fischer esterification. You certainly will encounter these two
reactions often in your study of organic chemistry.
17.8 Special Topic: Fatty Acids
We have spent many pages looking at the reactions of carboxylic acids. Now let’s
consider a class of molecules found extensively in Nature, called fatty acids.
These compounds contain long unbranched hydrocarbon chains with a single
carboxylic acid at one end. The properties of such molecules are strongly influ-
enced by the presence of both the long-chain and the acid group. Most fatty acids
in Nature are even-numbered long-chain acids because they are formed by a
process that puts the hydrocarbon chain together two carbons at a time using
an acetic acid derivative.
The fatty acids are usually found in plants and animals as the corresponding fat
or oil (Fig. 17.59), a fatty acid triester of glycerol. The fatty acid triesters that are
solids at room tempertature are called fats and those that are liquid at room tem-
perature are known as oils. The base-induced hydrolysis of these esters is called
saponification. We will learn more about this reaction in Chapter 18.
(CH
2
)
n
CH
3
2. H
3
O /H
2
O
+
1. KOH/H
2
O
+
A fat
Glycerol
n = 10 Lauric acid
n = 12 Myristic acid
n = 14 Palmitic acid
n = 16 Stearic acid
n = 18 Arachidic acid
..
..
O
..
..
O
..
..
..
O
(CH
2
)
n
CH
3
..
O
..
..
O
HO
3
..
..
O
(CH
2
)
n
CH
3
Fatty acid
WEB 3D
(CH
2
)
n
CH
3
..
..
O
..
..
OH
..
..
OH
..
..
..
..
OH
FIGURE 17.59 Some fatty acids that can be made from the hydrolysis of fats.
4
CoA stands for coenzyme A, one of many molecules necessary to promote an enzymatic reaction.
Fatty acids are most important molecules. For example, part of our body’s ener-
gy supply comes from a pathway that starts with the hydrolysis of a fat to fatty acids,
such as palmitic acid, which is then converted into a thioester (
4
).R
O
CO
O
SCoA
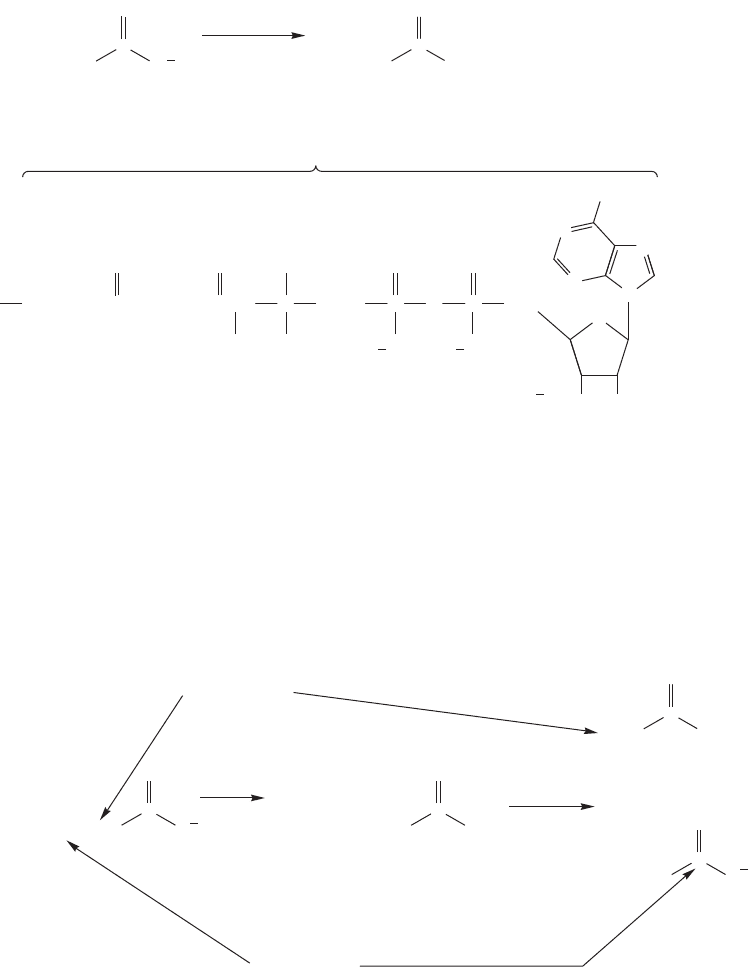
17.8 Special Topic: Fatty Acids 863
The thioester is repetitively cleaved by enzymes to give multiple two-carbon units
(Fig. 17.60). These acetyl groups are called acetyl-CoA, and are fed into the Krebs
cycle, a series of reactions in which acetyl-CoA is oxidized to CO
2
.
O O
O
O
C
C
C
CH
3
C
CH
3
CH CH
2
O
OH
O
O
O
OCH
2
O
HS CH
2
CH
2
NH
O
3
2
PO
OH
N
CH
2
CH
2
NH
N
N
N
8
Palmitate
Acetyl-CoA
NH
2
8 CoA-SH
CoA-SH
O
SCoA
CH
3
C
O
O
CH
3
(CH
2
)
14
P
P
CoA
FIGURE 17.60 Enzymes ultimately
convert fatty acids such as palmitate,
a 16-carbon fatty acid, into eight
molecules of acetyl-CoA.
CH
3
(CH
2
)
12
CH
2
CH
2
O
Palmitate
CoA-SH
Acetyl-CoA
SCoA
CH
3
(CH
2
)
12
O
Myristate
Same carbon
Same carbon
C
O
SCoA
CH
3
C
O
C
O
C
O
CH
3
(CH
2
)
12
CH
2
CH
2
βα
FIGURE 17.61 Fatty acids are deconstructed two carbons at a time. Note that the β carbon
of the palmitic acid becomes the carbonyl carbon of the 14-carbon acid.
Thus, it seems that the methyl group of acetyl-CoA must be the α carbon of
the original fatty acid and the β carbon of the fatty acid becomes the carbonyl car-
bon of myristate (Fig. 17.61). We will come back to this reaction in Chapter 19, but
you should be able to appreciate both the extent and difficulty of the task that Nature
faces here—How to break the α–β bond in palmitate? That’s no simple task, and
we shall need the material in Chapter 19 to solve it.
Fatty acids often have double bonds in the carbon chain. The presence of double
bonds in the triester fat affects their solubility and melting points. Saturated fats (those
having no double bonds) are solids, and there are well-documented health concerns
with diets that are high in saturated fat content. Fatty acids with trans double bonds
are also likely to be solid, one of the reasons for recent “trans-fat” health concerns.
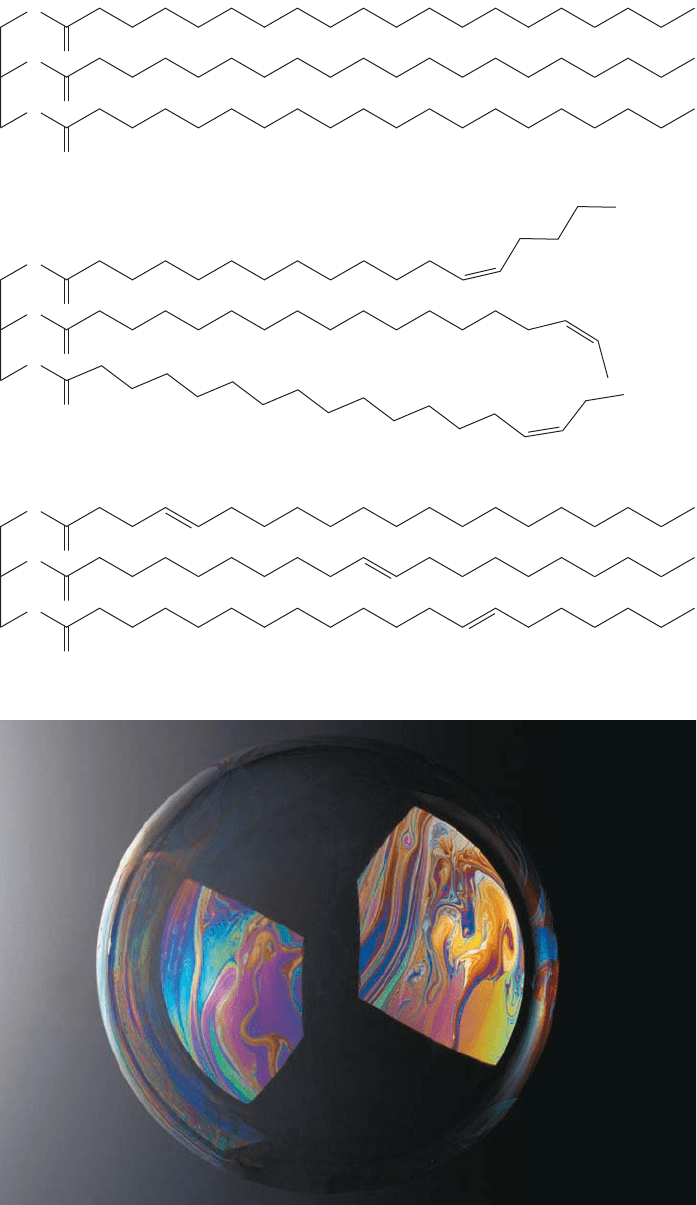
When fatty acids have cis double bonds, their corresponding triesters are oils, liq-
uids at room temperature. Because the cis double bond results in poorer stacking
between the chains, these molecules do not solidify as easily as their trans counter-
parts (Fig. 17.62).
864 CHAPTER 17 Carboxylic Acids
O
O
O
O
O
O
Saturated fat
O
O
O
O
O
O
cis Unsaturated fat (oil)
O
O
O
O
O
O
trans Unsaturated fat (trans fat)
FIGURE 17.62 The triesters with no
double bonds can stack much better
than the triesters with a cis double
bond.Trans fats also line up well
and have higher melting points than
cis fats.
Soap bubbles are thin films made
from long-chain carboxylic acid salts
that are aligned with each other.
17.8 Special Topic: Fatty Acids 865
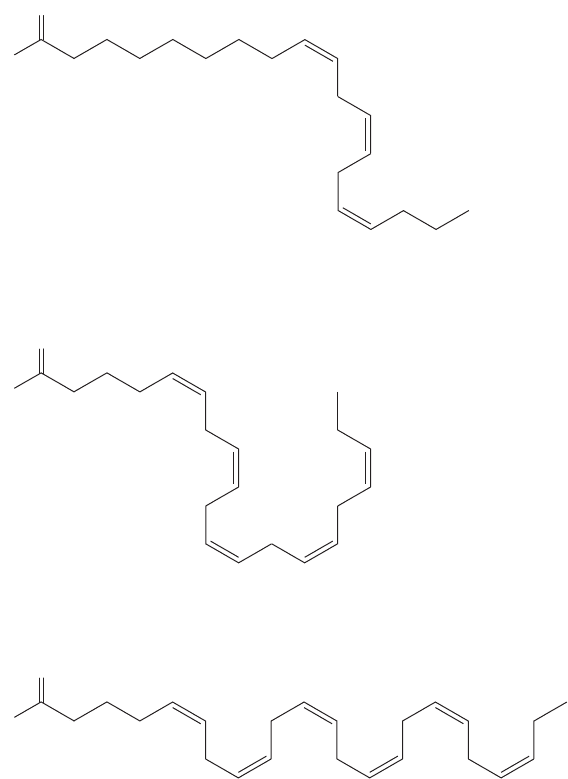
There are many fatty acids that are necessary for proper cellular function.
Humans make most of the fatty acids that are needed. However, the omega-3 fatty
acids (Fig. 17.63) are an essential part of our diet. Omega-3 fatty acids belong to
a family of long-chain carboxylic acids that have a cis double bond at the third
carbon from the end of the chain. The specific molecules differ in chain length
and in the number of additional double bonds in the chain.The FDA has report-
ed data that suggest omega-3 fatty acids reduce the risk of coronary heart disease.
O
HO
␣-Linolenic acid (ALA)
(Z, Z, Z )-9,12,15-Octadecatrienoic acid
(Z, Z, Z, Z, Z )-5,8,11,14,17-
Eicosapentaenoic acid (EPA)
(all Z )-4,7,10,13,16,19-
Docsahexaenoic acid (DHA)
O
HO
O
HO
FIGURE 17.63 The omega-3 fatty
acids involved in cellular function: α-
linolenic acid (ALA),
eicosapentaenoic acid (EPA), and
docosahexaenoic acid (DHA).
Omega-3 fatty acids are found in high concentrations in fish oil. Fish do not actu-
ally synthesize these compounds, but obtain them from algae. Even higher concen-
trations of omega-3 fatty acids are found in flaxseed oil. Lingonberry and kiwi are
also rich in these compounds.
866 CHAPTER 17 Carboxylic Acids
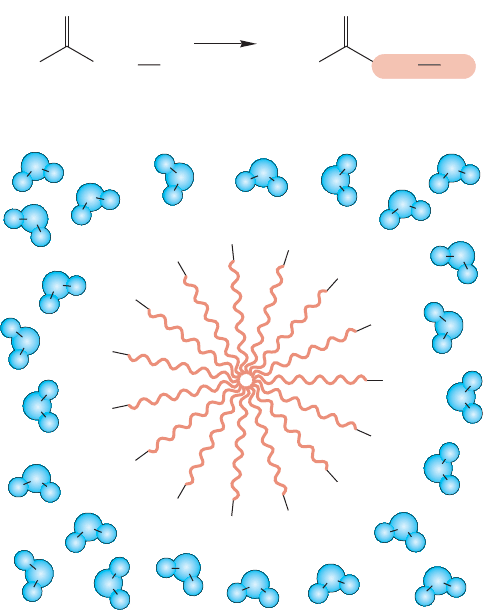
There is another topic of importance related to fatty acids. Soaps are the sodi-
um salts of long-chain fatty acids, and work in the following way.These compounds
contain both a highly polar end, the carboxylic acid salt, and a most nonpolar end,
the long-chain hydrocarbon end (Fig. 17.64). The polar end is soluble in polar
solvents such as water, but the nonpolar, hydrocarbon end is not. In aqueous solu-
tion,the hydrophobic (water-hating) hydrocarbon ends cluster together at the inside
of a sphere, protected from the aqueous environment by the hydrophilic (water-
loving) polar acid-salt groups forming the interface with the polar water molecules.
Such aggregates are called micelles. The cleaning ability of soaps comes from the
oil-capturing property of the hydrophobic portion of the micelle, which can remove
oil and grease from a surface.The micelle is soluble in water and so it washes away
the non-water-soluble oil and grease.
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
CO
O
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
Na
+
COO
–
A micelle
HO
O
..
..
(CH
2
)
n
CH
3
base
A fatty acid
O
(CH
2
)
n
CH
3
A soap
ONa
+
..
..
..
..
..
–
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
..
..
(b)
(a)
FIGURE 17.64 (a) The formation of a soap from a fatty acid.
(b) In polar media, soaps form micelles, with the hydrophobic
hydrocarbon chains directed toward the center of a sphere, away
from the polar solvent.The outside of the sphere contains the
polar carboxylate anions and their positive counterions.
Similar micellar species are formed from detergents, which are synthetic soaps.
One of the most common synthetic soaps is a long-chain sulfonic acid salt
(Fig. 17.65). Because detergents are industrially synthesized, they haven’t always
been biodegradable. Perhaps not surprisingly, the key to chemists finding an
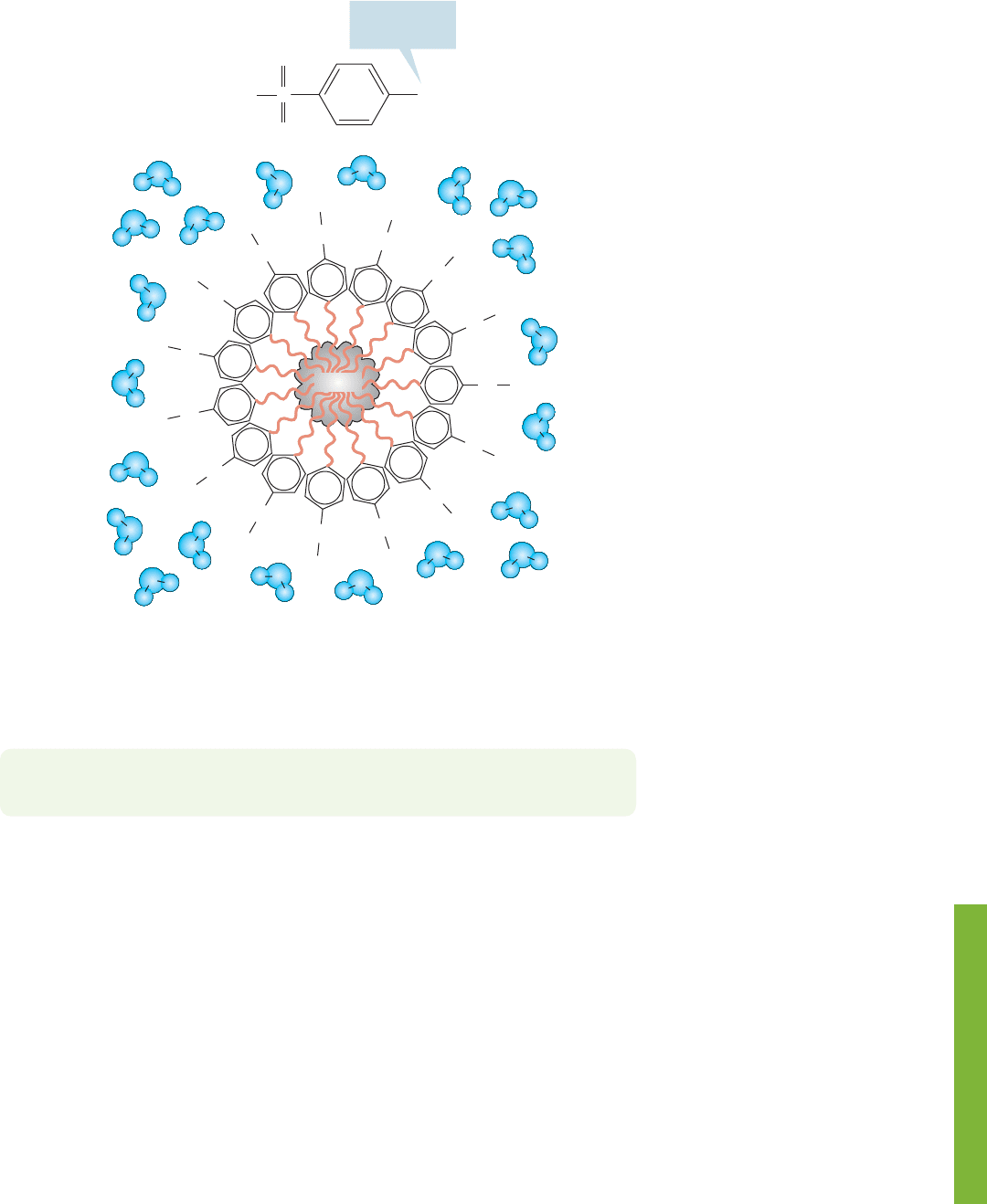
17.9 Summary 867
O
..
..
O
..
..
..
O
..
..
A long-chain
hydrocarbon
Na
+
–
S R
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
SO
2
O
–
Na
+
S
O
2
O
–
Na
+
SO
2
O
–
Grease
(b)
(a)
FIGURE 17.65 (a) A synthetic soap, a detergent, and (b) a micelle of
the detergent solubilizing a grease glob.
PROBLEM 17.24 What process do you suppose Nature uses to degrade the long-
chain detergent?
environmentally friendly soap was recognizing that Nature can oxidatively cleave
long-chain carbon compounds, but does not do as well with branched alkyl groups.
Using this “green” recognition has given us biodegradable detergents.
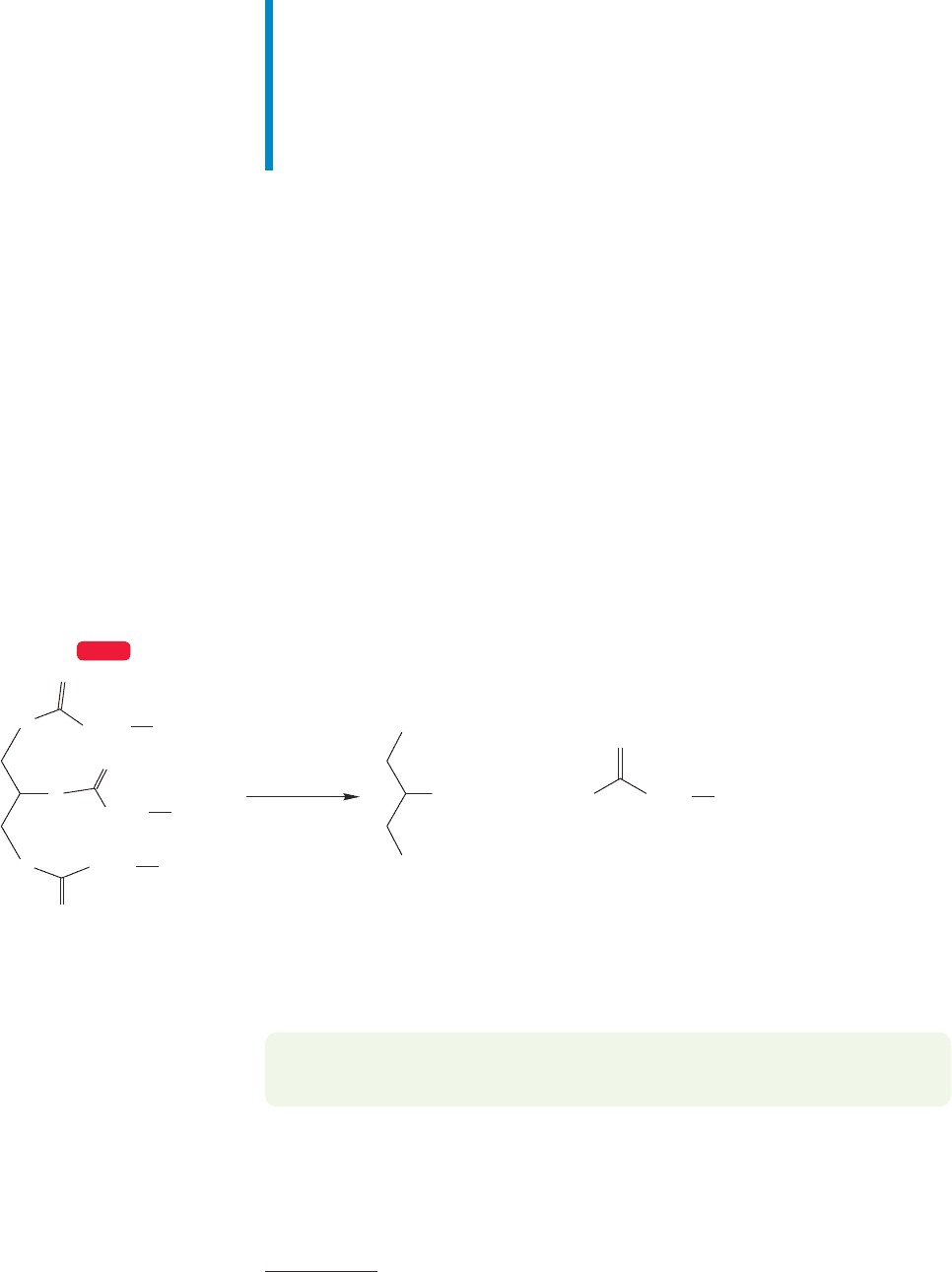
17.9 Summary
New Concepts
Carboxylic acids exhibit several kinds of reactivity. So far, we have
seen many kinds of molecules (aldehydes and ketones, for exam-
ple) that can be both electrophiles and nucleophiles. But car-
boxylic acids are even more polyreactive.They are Brønsted acids
through proton loss from the hydroxyl group. They are also elec-
trophiles (Lewis acids) through reaction at the carbonyl carbon.
Carboxylic acids act as Brønsted bases and nucleophiles (Lewis
bases) through reaction at the more basic carbonyl oxygen.
The Brønsted acidity of carboxylic acids, long thought to
rest largely upon the delocalization of the carboxylate anion
produced by ionization, is also dependent on the inductive
effect of the polarized carbonyl group.

868 CHAPTER 17 Carboxylic Acids
Most of the new reactions in this chapter are examples of
addition–elimination processes. This mechanism has appeared
occasionally before, but it is emphasized in this chapter for the
first time. Any carbonyl compound bearing a leaving group can
be attacked by a nucleophile to give a tetrahedral intermediate
(addition) that can then expel the leaving group (elimination)
(Fig. 17.66).
For carboxylic acids, the success of an addition–elimination
reaction depends on the transformation of the carboxyl hydroxyl
group into a better leaving group. This conversion is often done
through protonation in acid-catalyzed reactions. The best exam-
ple is Fischer esterification, and its cyclic variation, lactone for-
mation (Fig. 17.33).
The carboxyl OH can also be made into a better leaving
group through other chemical conversions. An excellent exam-
ple is acid chloride formation in which a chlorosulfite ester is
first produced. Addition of chloride to the carbonyl group of
the ester leads to an intermediate that can eliminate sulfur
dioxide and chloride, thus producing the acid chloride
(Figs. 17.42 and 17.43).
The acid chlorides contain an excellent leaving group,
and addition–elimination reactions are common (Fig. 17.44).
Occasionally, the carboxylate anion can be used as a displac-
ing agent in an S
N
2 reaction. In order for this reaction to be
successful, the Lewis acid partner in the reaction must be very
reactive (Fig. 17.30).
The electrochemical reaction of carboxylate anions
leads to hydrocarbons (Kolbe electrolysis). Other reactions
involving decarboxylations are briefly mentioned
(Fig. 17.54).
Key Terms
acid anhydride (p. 855)
activating agent (p. 850)
amide (p. 850)
carbamates (p. 860)
carbamic acid (p. 860)
carboxylate anion (p. 832)
decarboxylation (p. 858)
detergent (p. 866)
fatty acids (p. 862)
Fischer esterification (p. 841)
Hunsdiecker reaction (p. 861)
hydrophilic (p. 866)
hydrophobic (p. 866)
Kolbe electrolysis (p. 860)
lactam (p. 851)
lactone (p. 849)
micelle (p. 866)
ortho esters (p. 846)
saponification (p. 862)
soap (p. 866)
tetrahedral intermediate
(p. 842)
urea (p. 860)
Reactions, Mechanisms, and Tools
+
..
..
O
LR
C
addition
L
R
Nu
C
..
Nu
–
–
..
L
–
..
..
O
RNu
C
..
..
..
O
Tetrahedral intermediate
elimination
FIGURE 17.66 The addition–elimination reaction.
Syntheses
C
OH
R
O
C
Cl
R
O
SOCl
2
C
OH
R
O
C
Cl
R
O
PCl
5
A chlorosulfite
ester (an
activated acid) is
an intermediate
2. Acids1. Acid Halides
Other oxidizing agents (listed above) work
C
H
R
O
C
OH
R
O
HNO
3
[O] = KMnO
4
, HNO
3
, CrO
3
/H
2
O
K
2
Cr
2
O
7
/H
2
SO
4
, RuO
4
C
OH
R
O
CH
2
OHR
[O]
