Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

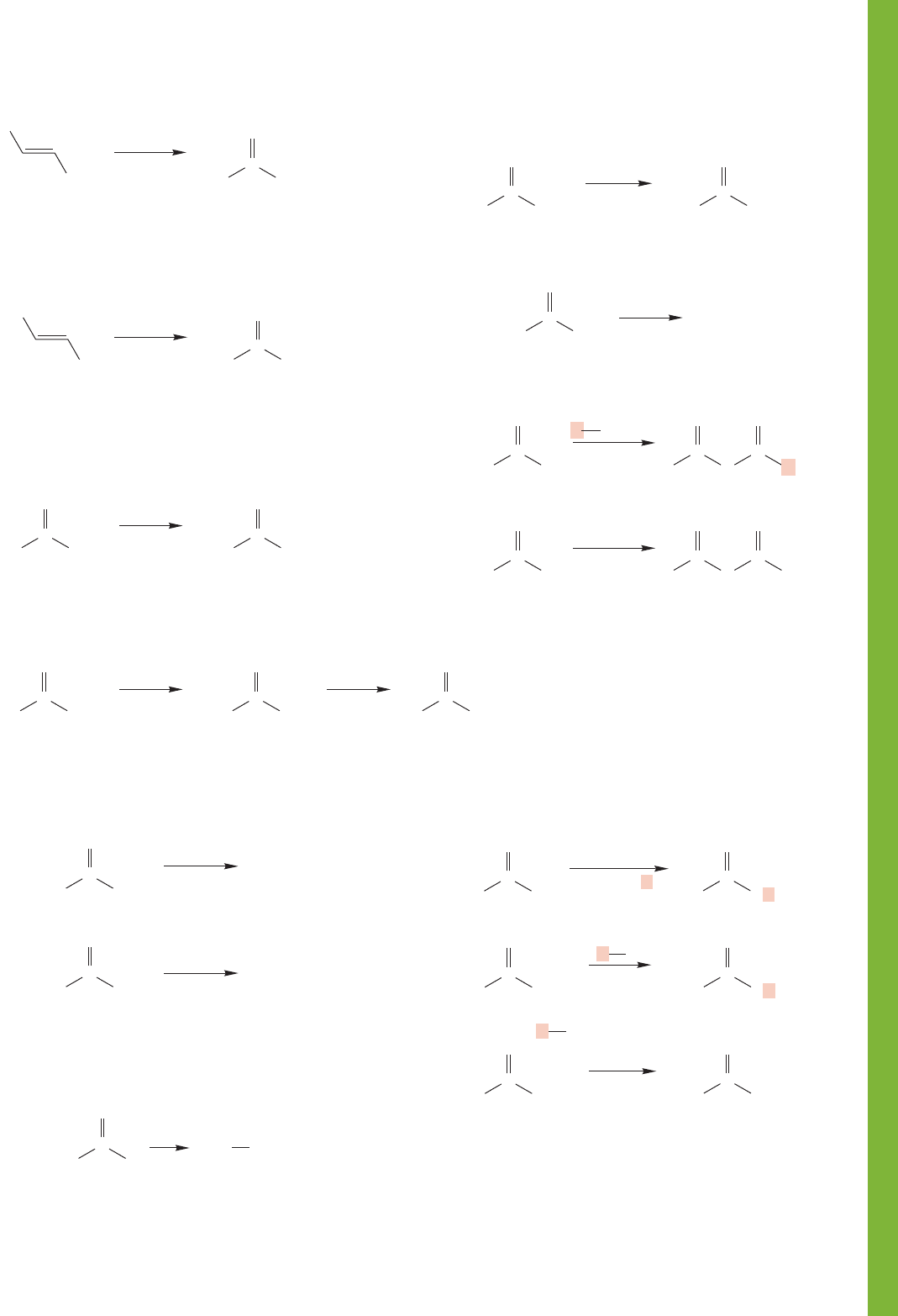
17.9 Summary 869
6. Amines
5. Amides (for cyclic amides, see “Lactams”)
4. Alkyl Halides
3. Alcohols
C
OR
R
O
C
O
–
R
O
C
OH
R
O
HO
–
H
3
O
+
H
2
O
Base-catalyzed ester hydrolysis
“saponification”
+
ROH
C
OR
R
O
C
OH
R
O
H
3
O
H
2
O
Acid-catalyzed ester hydrolysis
+
C
OH
R
2
O
KMnO
4
18-crown-6
The crown ether is essential, as it
brings the KMnO
4
into solution
R
R
H
3
O
+
Note the oxidative workup; the aldehyde can
be obtained by using dimethyl sulfide or H
2
/Pd
C
OH
R
2
O
1. O
3
2. H
2
O
2
R
R
C
OH
R
O
1. LiAlH
4
2. H
2
O
RCH
2
OH
An aluminum alkoxide is a leaving group in this reaction
C
OH
RO
O
ROH
Δ
Decarboxylation of a bicarbonate ester
See also other “hydroxy” compounds in this section
Br
2
RCH
2
C
O
–
Ag
+
O
RCH
2
Br + CO
2
+ AgBr
Δ
The Hunsdiecker reaction is used
almost exclusively for bromides
C
OH
R
O
C
NR
2
R
O
NHR
2
DCC
An addition–elimination process
C
NR
2
HO
O
Decarboxylation of a carbamic acid
Δ
HNR
2
+ CO
2
7. Anhydrides
C
Cl
R
O
C
O
R
O
C
O
R COOH
R
This addition–elimination process works even
better with the carboxylate anion as nucleophile
C
OH
R
O
C
O
RR
O
C
O
P
2
O
5
Other dehydrating agents also work; intramolecular
reactions can often be accomplished by simple heating
8. Esters (for cyclic esters, see “Lactones”)
C
O
–
R
O
C
OR
R
O
R I
For this S
N
2 reaction to succeed,
R I must be very reactive
C
OH
R
O
C
OCH
3
R
O
CH
2
N
2
Only common for methyl esters
C
OH
R
O
C
ORR
O
acid
excess HOR
Fischer esterification
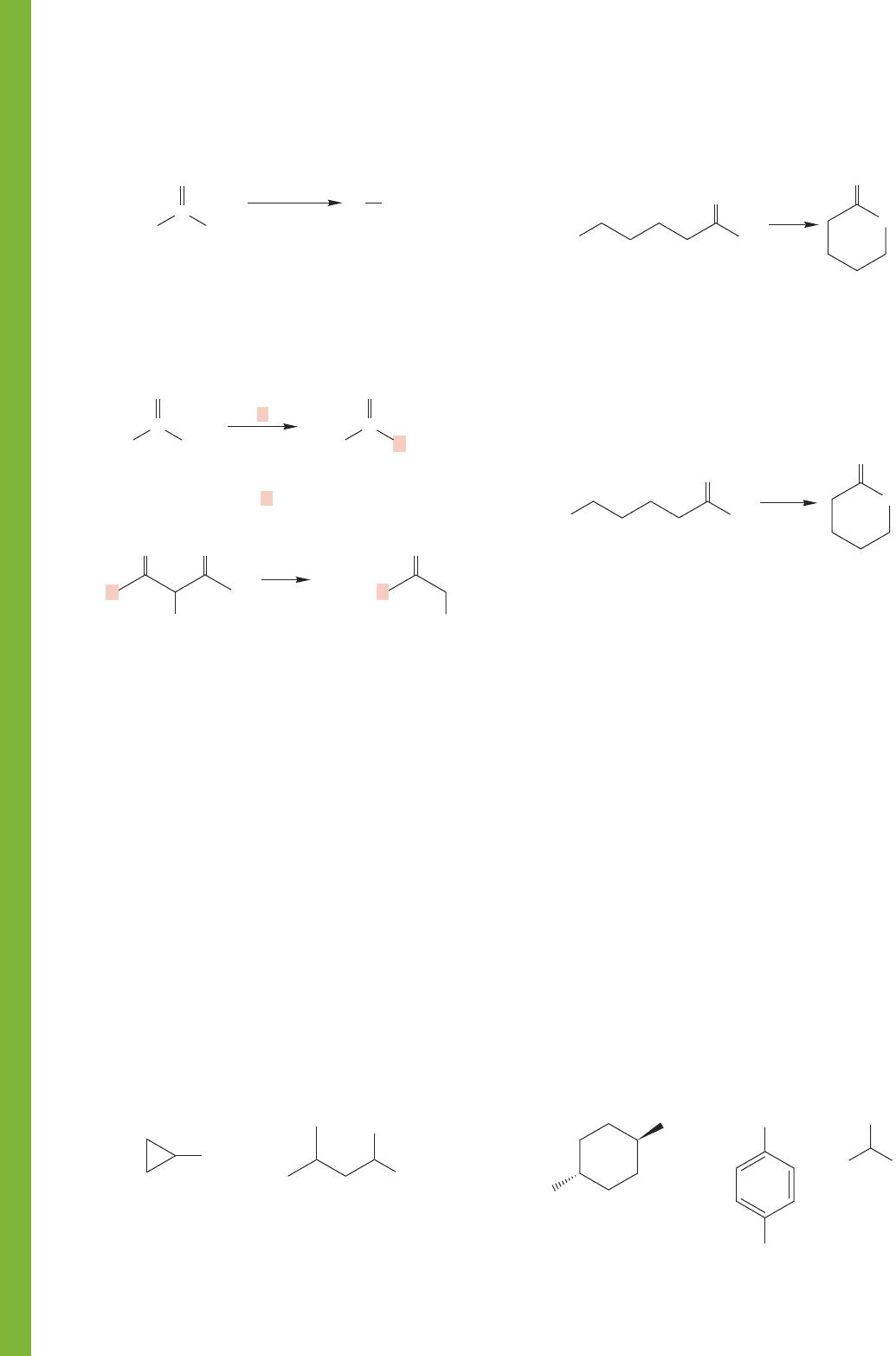
870 CHAPTER 17 Carboxylic Acids
11. Lactams
Addition–elimination reactions are straightforward: A nucleo-
phile adds to the carbonyl carbon, then the carbonyl is reconsti-
tuted as a leaving group is displaced. But there is such variety!
That’s where the difficulty lies—in seeing through all the
“spinach” hanging on the nucleophile and hiding the carbonyl—
to find the simple mechanism at the heart of matters.
Also, there are vast numbers of small steps—proton trans-
fers mostly—in these mechanisms, and that makes it more
challenging to get them all right.
Finally, there is a small error that all of us seem to make
on occasion. This mistake is to add an electrophile to the
wrong oxygen of a carboxylic acid. It is the carbonyl oxygen
that is the more nucleophilic and basic site, not the hydroxyl
oxygen.
12. Lactones
10. Ketones
9. Hydrocarbons
C
O
–
R
O
electrolysis
The Kolbe electrolysis, a radical dimerization process
R + CO
2
R
2
OH
O
C
OH
R
R
O
R
C
R
O
1. 2 RLi
2. H
2
O
Δ,
acid
Decarboxylation of β-keto acids
The hydrate is an intermediate,
2 moles of RLi are required
R
O O
R
R
CO
2
+
This formation of cyclic amides works
best for unstrained rings
H
2
N
OH
O
DCC
NH
O
Intramolecular Fischer esterification
HO
OH
O
H
3
O
O
O
+
Common Errors
17.10 Additional Problems
PROBLEM 17.25 Write names for the following carboxylic
acids:
(c) (d) (e)
CH(CH
3
)
2
H
2
N
COOH
COOH
COOH
HO
Cl
(a) (b)
COOH
COOH
Br
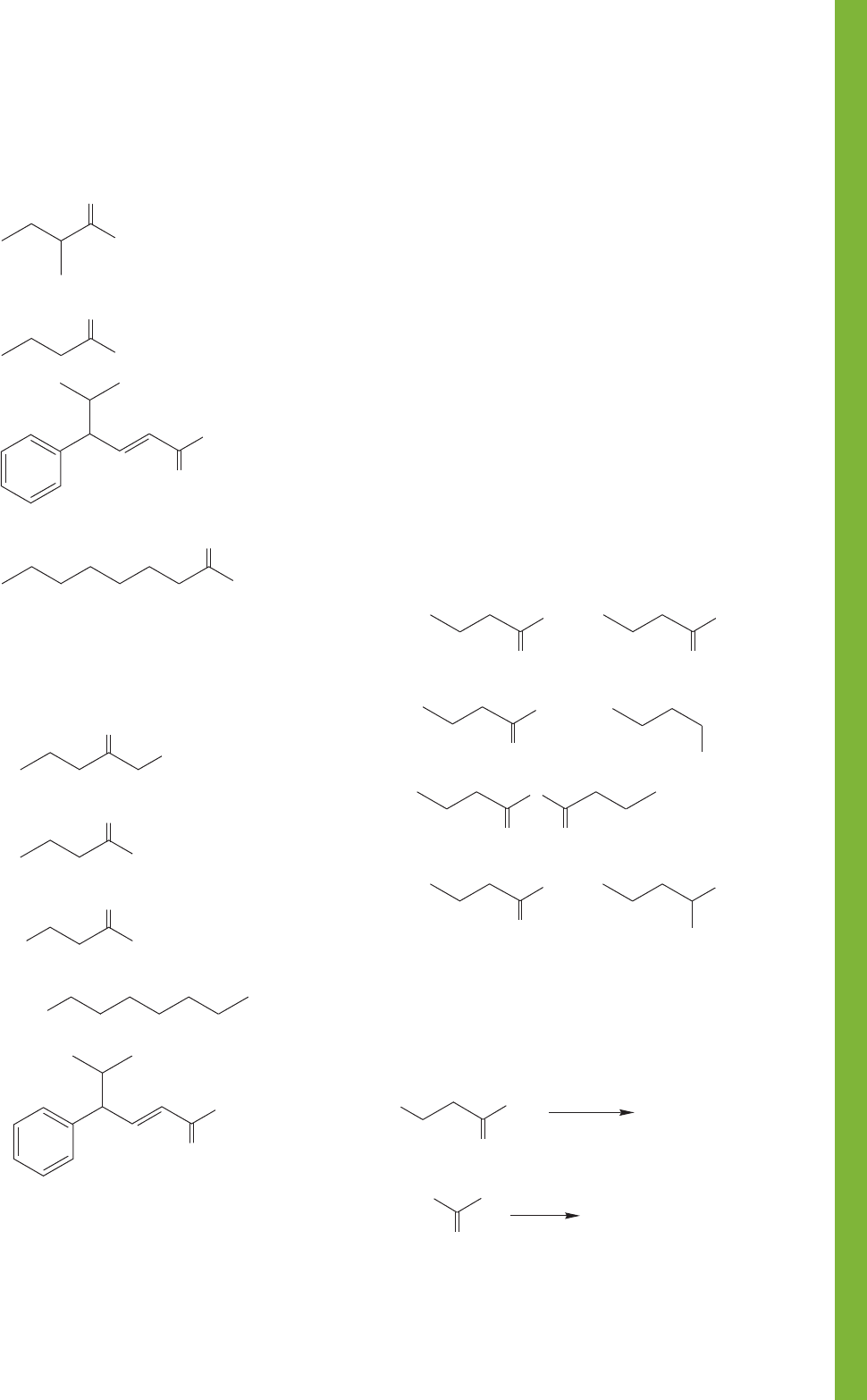
17.10 Additional Problems 871
PROBLEM 17.28 Draw a structure for each of the following
molecules:
(a) 2-aminopropanoic acid (alanine)
(b) 2-hydroxypropanoic acid (lactic acid)
(c) (Z)-2-methyl-2-butenoic acid
(d) 2-hydroxy-1,2,3-propanetricarboxylic acid (citric acid)
(e) 2-hydroxybenzoic acid (o-hydroxybenzoic acid or salicylic
acid)
(f) 2-oxopropanoic acid (pyruvic acid)
PROBLEM 17.29 Draw a structure for each of the following
compounds:
(a) lithium 2-hydroxy-3-methylpentanoate
(b) sodium benzoate
(c) sodium 2-amino-3-phenylpropanoate
(d) potassium acetate
(e) potassium 3-butenoate
PROBLEM 17.30 Give reagents for converting butanoic acid
into the following compounds:
PROBLEM 17.27 Provide the IUPAC name for the following
compounds:
PROBLEM 17.26 Write names for the following carboxylate
salts:
O
(a)
Na
+
O
–
O
(b)
Li
+
O
–
(d)
K
+
O
–
O
O
(c)
Na
+
O
–
(d)
HO
2
C
(c)
Ph OH
O
O
(b)
OH
O
O
(a)
OH
(e)
OH
(a) (b)
(c)
(d)
(e)
OCH
3
O O
O
NHCH
3
Cl
O
O
OH
O
(f) (g)
CH
3
O
CH
3
OH
PROBLEM 17.31 Give the major organic products expected in
each of the following reactions:
(b)
RCH
2
O
2
OH
DCC
CH
3
CN
(a)
OH
O
CH
3
OH
2
CH
3
OH, Δ
Br
+
(continued)
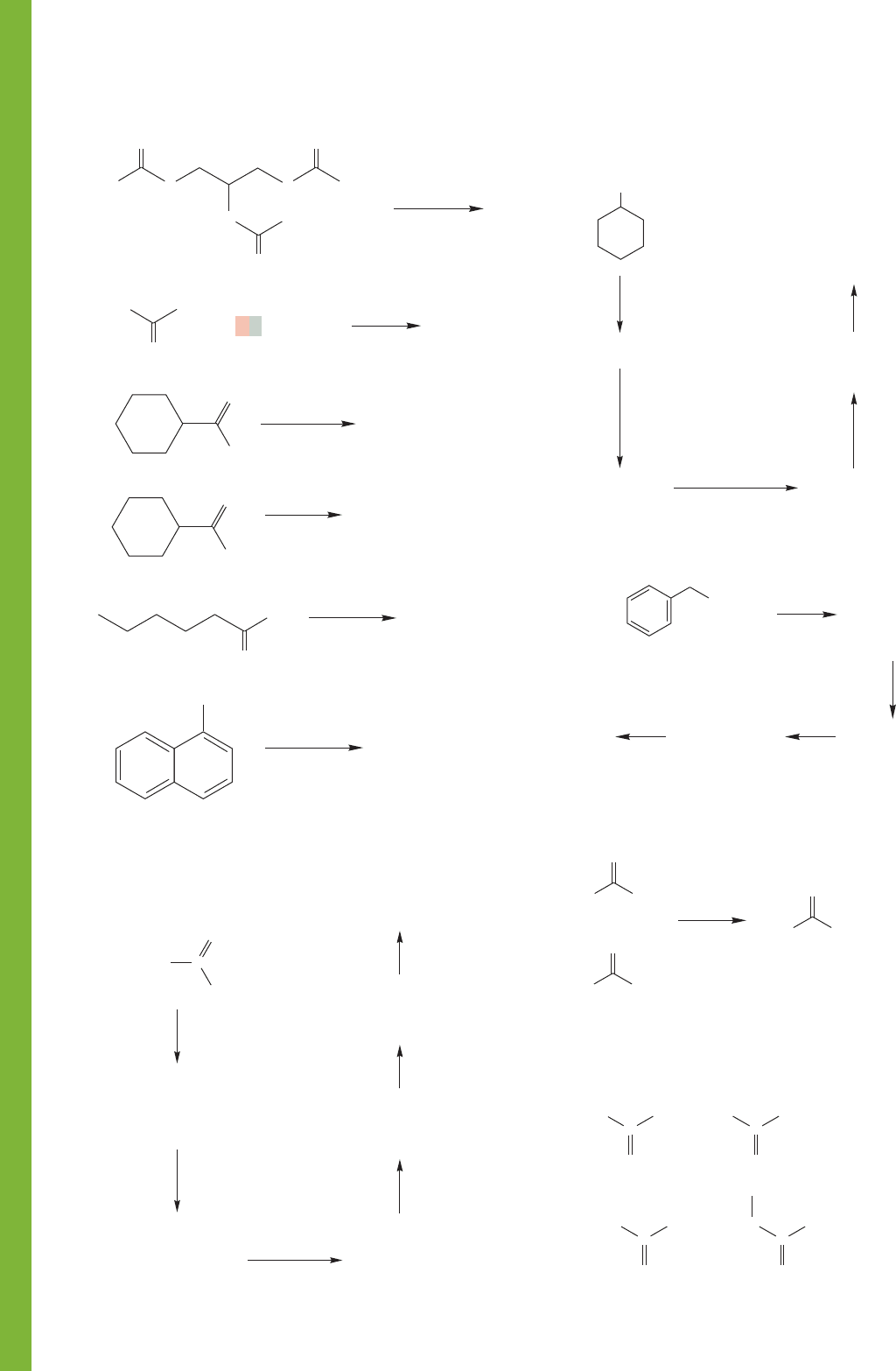
872 CHAPTER 17 Carboxylic Acids
PROBLEM 17.35 Provide a mechanism for this unusual esterifi-
cation process.
PROBLEM 17.34 Provide structures for compounds A–D.
PROBLEM 17.33 We begin this problem with cyclohexanol.
Your job is to determine the structures of the compounds A–D.
PROBLEM 17.32 Provide structures for compounds A–F.
Mechanisms are not required, but may be helpful in your analysis.
(g)
OH
O
(h)
(f)
O
OH
1. SOCl
2
2. Benzene
AlCl
3
1. NaH
2. CH
3
Li
3. H
2
O
1. Mg, ether
2. CO
2
(c)
O
O
OO
O
1. NaOH/
H
2
O, Δ
C
13
H
27
C
13
H
27
C
13
H
27
O
2. H
3
O/H
2
O
+
3. H
2
O/H
3
O
+
(d)
RCH
2
O
OH
DCC
CH
3
CN
RRCHNH
2
+
+
(e)
O
OH
1. NaH
2. H
3
O/H
2
O
Br
(CH
3
)
3
C
CH
2
N
2
A
(C
6
H
12
O )
2
KOH
H
2
O
B
(C
5
H
9
O
2
–
+
K)
(DMSO)
C
(C
6
H
12
O )
2
H
3
O
+
H
2
O
D
(C
5
H
10
O )
2
SOCl
2
E
(C
5
H
9
OCl)
CH
3
OH
F
(C
6
H
12
O )
2
CH I
3
C
O
OH
A
B
(C
7
H
12
O )
2
1. LiAlH
4
2. H
2
O/ H
3
O
+
C
(C
7
H
14
O)
3. H
3
O
+
/H
2
O
B
H
3
O
+
H
3
O
+
/H
2
O
D
(C
14
H
24
O )
2
HBr
OH
B + C
2
1. Mg
2. CO
COOH SOCl
2
A
(C
8
H
7
ClO)
NBS
HBr
B
(C
8
H
6
BrClO)
H
2
O
C
NH
3
D
(C
8
H
9
NO )
2
(C
8
H
7
BrO
2
)
H
3
C
OH
O
H
3
C
H
3
O
3
OC(CH
3
)
O
H
3
CCH
3
CH
2
+
+
PROBLEM 17.36 Provide brief explanations for the pK
a
differ-
ences noted in the following:
(a)
C
OH
O
H
C
OH
O
H
3
C
3.77 4.76
(b)
C
OH
O
H
2
C
1.68
C
OH
O
H
3
C
4.76
NO
2
17.10 Additional Problems 873
PROBLEM 17.42 There is a compound called the Vilsmeier
reagent (we will ask you about its formation in Chapter 18) that
reacts with carboxylic acids to give acid chlorides and the molecule
dimethylformamide (DMF). Provide a mechanism for this process.
PROBLEM 17.41 In Problem 17.14, you worked out the mecha-
nism for lactone formation from an hydroxy acid. Here is the
related reaction, the formation of a cyclic amide, a lactam.
Provide a mechanism.
PROBLEM 17.37 In Section 17.7b, we saw how dicyclohexyl-
carbodiimide (DCC) could be used as a dehydrating agent for
the formation of amides from carboxylic acids and amines.
Another reagent that can be used to good effect is the phosgene
derivative N,N ′-carbonyldiimidazole (CDI). Carboxylic acids
and CDI react under mild conditions to form imidazolides, 1,
which then easily react with amines to give amides. Propose
mechanisms for the formation of 1 from carboxylic acids and
CDI, and for the formation of amides from 1 and amines.
(c)
(d)
4.19 4.88
6.23 4.38
1.92
3.02
COOH
COOH
COOHHOOC
COOH
HOOC
(e)
COOHOOC
COO
HOOC
–
–
CDI
THF
RRNH
25 ⬚C
1
R
OH
+
C
O
N
N
N
N
O
O
R
N
N
R
N
C
O
R
R
+
O
O
COOH
COOH
O
CH
3
H
3
C
O
O
O
O
H
3
C
OH
2
COOH
H
2
N
acid catalyst
H
A lactam
(97%)
N
O
25 ⬚C, 5 h
C
C
OH
R
(CH
3
)
2
N
++
+
Cl
O
C
Cl
–
Cl
R
O
H
C
DMFVilsmeier reagent
(CH
3
)
2
N
H
O
PROBLEM 17.38 Dicyclohexylcarbodiimide (DCC) can also be
used to activate a carboxylic acid in the synthesis of esters.
Show how you would use DCC to synthesize cyclohexyl acetate
starting with any alcohols.
PROBLEM 17.39 Show the mechanism for the esterification
reaction between pentanoic acid and ethanol using DCC.
PROBLEM 17.40 Another method of forming an anhydride is
to allow a dicarboxylic acid to react with another anhydride
such as acetic anhydride, Ac
2
O. Propose a mechanism for the
reaction in the next column.
PROBLEM 17.43 Show the product that would be obtained
from the decarboxylation of each of the following compounds:
(a)
HO
2
C
(c)
HO
2
C CO
2
H
O
(b)
Ph OH
OO
(d)
CO
2
H
O
874 CHAPTER 17 Carboxylic Acids
PROBLEM 17.48 Explain the following observations. Note that
the only difference in the starting materials is the stereochem-
istry of the carbon–carbon double bond.
PROBLEM 17.47 Propose an arrow formalism mechanism for the
following reaction. Hint: See Section 17.7g and Figure 17.57.
PROBLEM 17.46 Propose syntheses of the following molecules
from the readily available fatty acid, lauric acid (1):
PROBLEM 17.45 An imide is a functional group similar to an
acid anhydride. Instead of an oxygen between two carbonyls, an
imide has a nitrogen between two carbonyls. Suggest a method
for making the imide shown below starting with pentanedioic
acid and benzyl amine.
PROBLEM 17.44 Predict the product for each of the following
reactions:
(c)
HO
2
C
O
(d)
O
OHO
O
(a)
OH
(b)
CO
2
H
SOCl
2
benzene
SOCl
2
benzene
O
(e)
OH
HO
OHO
excess
SOCl
2
benzene
SOCl
2
benzene
SOCl
2
benzene
Br
O
N Ph
O
CH
3
(CH
2
)
10
COOH
(a) CH
3
(CH
2
)
11
Br
(c) CH
3
(CH
2
)
11
COOH
(b) CH
3
(CH
2
)
10
Br
1
O
COOH
COOH
CH
3
CH
3
KOH
CH
3
OH
CH
3
C
6
H
13
H
3
C
electrolysis
+
O
+
O
O
COOCH
3
CH
3
CH
3
OH
2
CH
3
OH
H
3
C
HO
OH
COOCH
3
O
O
COOCH
3
CH
3
+
CH
3
OH
2
CH
3
OH
H
3
C
O
HO
O
(a) (b)
(c) (d)
COOH
?
O
O
O
O
N
H
PROBLEM 17.49 Here are some somewhat more complicated
syntheses. Devise ways to make the compounds shown below.
You may use cyclopentanecarboxylic acid as your only source
of carbon.
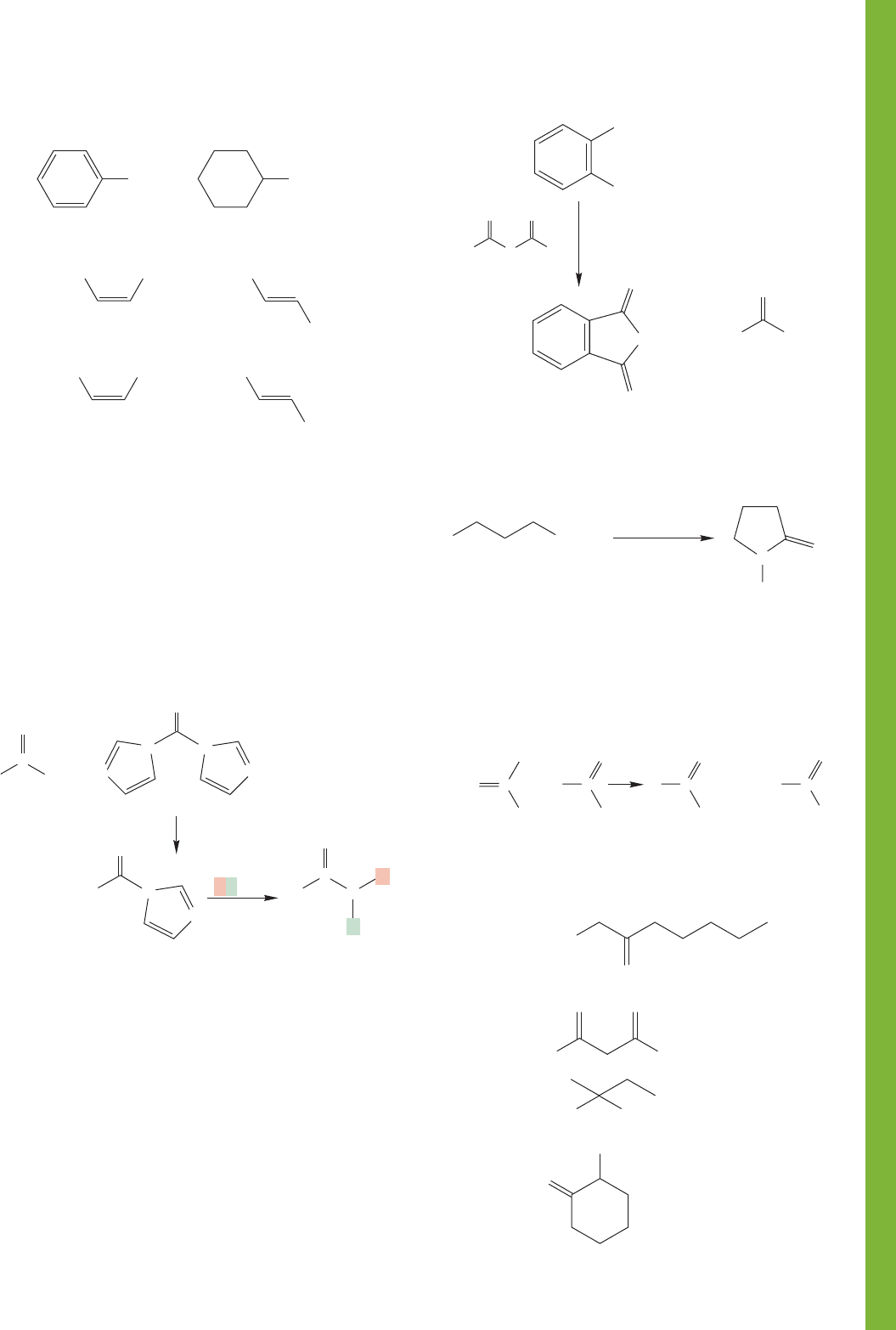
17.10 Additional Problems 875
1 2 3
+
O
O
O
Δ
H
2
O
Cyclo-
pentadiene
Maleic
anhydride
2. H
2
O
1. conc.
H
2
SO
4
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 17.53 Select the reaction “Fischer esterification”
and click on the play button. Explain why the first step of the
reaction, protonation of acetic acid, occurs at the oxygen that is
shown. How does the LUMO track of the first intermediate
help explain this selectivity.
PROBLEM 17.54 Observe the “Acid chloride formation” ani-
mation. What is the shape of thionyl chloride? What is the
shape of the SO
2
that is formed at the end of the reaction? This
reaction converts a carboxylic acid into a much more reactive
acid chloride. Why does the energy diagram show the reaction
as being exothermic?
PROBLEM 17.55 The reaction titled “Ester hydrolysis” is also
known as saponification. How many intermediates are there in
this reaction? Why does the tetrahedral intermediate eliminate
the methoxy group so rapidly in this animation?
Compound 3
IR (KBr): 3450–2500 (br, s), 1770 (s), 1690 (s) cm
1
1
H NMR (CDCl
3
): δ 1.50–1.75 (m, 3H)
1.90–2.05 (m, 1H)
2.45–2.55 (m, 1H)
2.65–2.75 (m, 1H)
2.90–3.05 (m, 1H)
3.20–3.30 (m, 1H)
4.75–4.85 (m, 1H)
12.4 (s, 1H)
PROBLEM 17.52 When N-nitroso-N-methylurea (NMU) is
treated with a two-phase solvent system, H
2
O/KOH and ether,
the yellow color of diazomethane (p. 848) rapidly appears in the
ether layer. Show the mechanism.
PROBLEM 17.50 Estragole (1) is a major constituent of
tarragon oil. Treatment of 1 with hydrogen bromide in the
presence of peroxides affords 2. Compound 2 reacts with Mg
in ether, followed by addition of carbon dioxide and acidifica-
tion, to give 3. Spectral data for compound 3 are summarized
below. Deduce the structure of compound 3 and propose
structures for estragole and compound 2.
Compound 3
Mass spectrum: m/z 194 (p)
IR (Nujol): 3330–2500 (br, s), 1696 (s) cm
1
1
H NMR (CDCl
3
): δ 1.95 (quintet, J 7 Hz, 2H)
2.34 (t, J 7 Hz, 2H)
2.59 (t, J 7 Hz, 2H)
3.74 (s, 3H)
6.75 (d, J 8 Hz, 2H)
7.05 (d, J 8 Hz, 2H)
11.6 (s, 1H)
PROBLEM 17.51 Reaction of cyclopentadiene and maleic
anhydride affords compound 1. Hydrolysis of 1 leads to 2,
which gives an isomeric compound 3 upon treatment with
concentrated sulfuric acid. Spectral data for compounds
1–3 are shown below. Propose structures for compounds
1–3 and provide mechanisms for their formations.
N-Nitroso-N-methylurea
(NMU)
Diazomethane
(as a yellow solution in ether)
CH
2
N
2
KOH
H
2
O/ether
C
CH
3
H
2
N
N
N
O
O
Compound 1
Mass spectrum: m/z 164 (p, 3%), 91 (23%), 66 (100%),
65 (22%)
IR (Nujol): 1854 (m) and 1774 (s) cm
1
1
H NMR (CDCl
3
): δ 1.60–1.70 (m, 1H)
3.40–3.50 (m, 1H)
3.70–3.80 (m, 1H)
6.20–6.30 (m, 1H)
Compound 2
IR (Nujol): 3450–2500 (br, s), 1710 (s) cm
1
1
H NMR (CDCl
3
): δ 0.75–0.95 (m, 1H)
2.50–2.60 (m, 1H)
2.70–2.80 (m, 1H)
5.60–5.70 (m, 1H)
11.35 (s, 1H)

Derivatives of Carboxylic
Acids: Acyl Compounds
876
18.1 Preview
18.2 Nomenclature
18.3 Physical Properties and
Structures of Acyl Compounds
18.4 Acidity and Basicity of Acyl
Compounds
18.5 Spectral Characteristics
18.6 Reactions of Acid Chlorides:
Synthesis of Acyl Compounds
18.7 Reactions of Anhydrides
18.8 Reactions of Esters
18.9 Reactions of Amides
18.10 Reactions of Nitriles
18.11 Reactions of Ketenes
18.12 Special Topic: Other Synthetic
Routes to Acid Derivatives
18.13 Special Topic: Thermal
Elimination Reactions of
Esters
18.14 Special Topic: A Family of
Concerted Rearrangements
of Acyl Compounds
18.15 Summary
18.16 Additional Problems
18
TART! The flavors and fragrances of fruit come from volatile esters that we taste
and smell.
18.1 Preview 877
OH
O
..
..
Cl
..
..
..
..
..
OR¿
R¿
R
R
CC
OO
C
Carboxylic acids
R
O
O
..
..
..
..
C
Esters
R
R¿
NH
2
O
..
..
..
O
..
..
..
..
..
..
..
LR
C
O
..
..
..
..
C
Amides
Nitriles
(cyanides)
Ketenes
Acyl-like compounds
Acyl compounds
R
CCCNR
O
..
..
C
Acid chlorides
R
Anhydrides
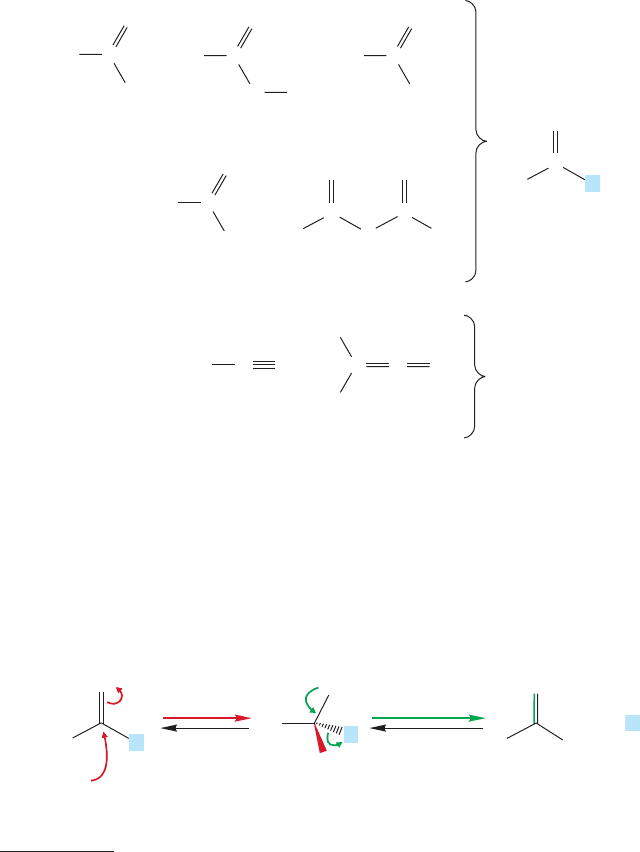
FIGURE 18.1 Some acyl and related
derivatives of carboxylic acids.
FIGURE 18.2 The most important
mechanism for reactions of
acyl compounds is the
addition–elimination process.
Strange shadows from the flames will grow,
Till things we’ve never seen seem familiar . . .
—JERRY GARCIA AND ROBERT HUNTER,
1
TERRAPIN STATION
18.1 Preview
In this chapter, we focus on things that should seem familiar even though we have
never seen them in detail. Many of the molecules we saw in Chapter 17 will reap-
pear, for example.Acid halides, anhydrides,esters,and amides are all acyl compounds
of the general structure . These compounds are also known as acid
derivatives, because historically they were first derived from carboxylic acids. Many
are shown in Figure 18.1 along with the related nitriles and ketenes. This chapter
will deal with all six of these functional groups (acid halides, anhydrides, esters,
amides, nitriles, and ketenes). The nitriles and ketenes are included in this discus-
sion because, like acid derivatives, they undergo reaction with water to form a
carboxylic acid. These six functional groups are at the same oxidation level.
R
O
CO
O
L
1
Jerry Garcia (1942–1995) played lead guitar with the Grateful Dead, and Robert Hunter (b. 1941) was a
frequent songwriting collaborator.
Not only are these molecules related by having similar structures, but their
chemistries are also closely intertwined—a reaction of one acyl compound is generally
a synthesis of another,for example.The unifying properties of these seemingly disparate
compounds allow us to learn their chemistry as a group.The structures of the acyl com-
pounds are determined largely by the interaction between the L group and the carbonyl,
and the chemistry of these acid derivatives is dominated by addition–elimination
reactions in which the L group is replaced with some other substituent (Fig. 18.2).
+
Nu
..
–
Nu
Nu
Tetrahedral
intermediate
..
–
–
–
L
L
L
addition
of
..
–
elimination
of L
R
R
Nu
R
O
..
..
O
..
..
..
O
..
..
..

878 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
Note the tetrahedral intermediate at the center of this generic mechanism.This addi-
tion–elimination process is an equilibrium, and can proceed in both the “forward”and
“backward” directions.The old leaving group L
is also a nucleophile.
The acid derivatives deserve our full attention.They are all around us in life. Esters
are of utmost importance to the fragrance and flavoring industry.The sweet odors of fruits
and perfumes are usually a result of volatile esters.Amides are found throughout biochem-
istry. It is the amide group that defines enzyme structure, which in turn defines us. We
will cover more of the bio-organic chemistry of amides in Chapter 23.
In this chapter we will first go over some common ground—nomenclature,struc-
ture, and spectra—and then go on to examine reactions and syntheses for the differ-
ent derivatives.
The chemistry in the next two chapters can be either daunting or exhilarating.
Much depends on how well you have the basic reactions understood.If you do under-
stand them, you will be able to apply them in the new situations we are about to
explore.If not,the next chapters will seem crammed with new and complicated reac-
tions, each of which requires an effort to understand. Like anything else, learning
chemistry can have a strong psychological component. When you are able to think,
“That’s not so bad, it’s just an application of ...,”you gain confidence and what
you see on the page is likely to stick in your mind.
At this point in your study of organic chemistry, it is possible to ask questions
that stretch your knowledge and talent. It’s hard to teach this kind of problem solv-
ing, because success has a lot to do with having worked lots of related problems in
the past. Nevertheless, there are some techniques that we can try to pass on. Don’t
worry if you don’t get every problem! Our colleagues can write problems that we
will have difficulty with. It’s no disgrace to have trouble with a problem. There are
several favorite problems that we’ve been working on (not continuously) for many
years. You’ll see some of them. Success in the chemistry business has relatively lit-
tle to do with how fast you can solve problems, at least not problems that require
thought and the bringing together of information from different areas.People think
in different ways and therefore answers to problems appear at different rates. There
are those who are blindingly fast at this kind of thing and others who reach answers
more slowly. There is nothing wrong with chewing over a problem several times,
approaching it in several ways. Letting a particularly vexing problem rest for a while
is often a good idea. Of course, this is the bane of exam writers (and even more so of
exam answerers). How do you write an exam that allows for thought and doesn’t
simply favor the student who can answer quickly? It’s hard, especially if you don’t
have the luxury of asking a small number of questions and giving lots of time.
What does make for success in the real world of organic chemistry? Nothing
correlates better than love of the subject,and by “subject”we do not necessarily mean
what you are doing now, but rather really doing organic chemistry. Doing organic
chemistry means not only liking the manipulative aspects of the subject, although
that is a great help, but also loving the questions themselves. At any rate, it is not the
number of A’s on your transcript that correlates best with your later success in life!
ESSENTIAL SKILLS AND DETAILS
1. As always, we need to be able to generalize.This chapter describes the chemistry of a set of
related acyl compounds. It will be very hard to memorize the vast array of addition–
elimination processes, but it should be relatively easy to see them all as the same reaction
repeated over and over. Only the detailed structure changes—the overall reaction does not.
2. It will be helpful to keep in mind the order of reactivity that acid derivatives have with
respect to nucleophiles: acid chlorides anhydrides esters amides.
