Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

18.2 Nomenclature 879
3.
Amides are not like amines. Students often equate the two functional groups, but they
are very dissimilar. You should be able to recognize their differences and explain why
these compounds are so different.
4. Carbon–nitrogen triple bonds (nitriles) undergo addition reactions, as do carbonyl
compounds.
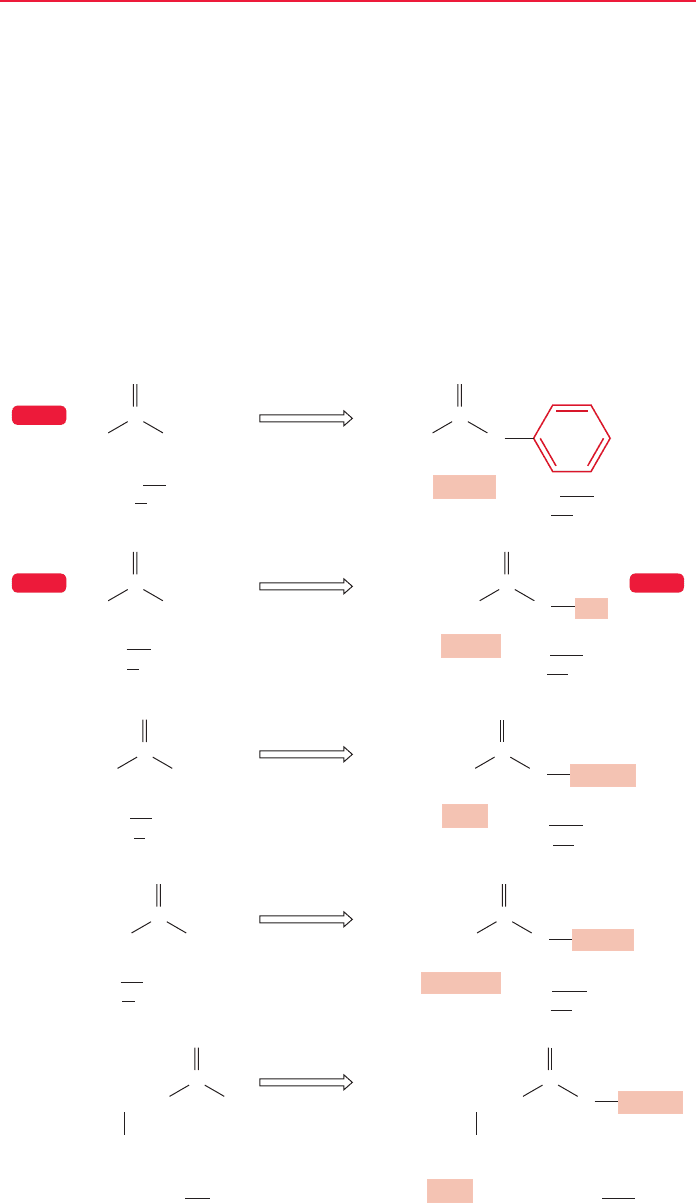
18.2 Nomenclature
The names of acyl compounds are based on the parent carboxylic acid.The IUPAC
systematic naming protocol for esters ( ) has the R group named as the
carboxylic acid and the ic acid is replaced with the suffix ate.So the root word becomes
alkanoate.The R′ group is named as the appropriate alkyl group and it is written as
a separate word in front of the alkanoate. Therefore, the generic ester is alkyl
alkanoate.The carbonyl carbon of the ester gets top priority in the naming scheme
and substituents are numbered with respect to the carbonyl carbon as position 1
(Fig. 18.3). The common names for methanoate and ethanoate are formate and
acetate, respectively, so we will frequently encounter alkyl (methyl, ethyl, etc.) for-
mates and acetates.
R
O
COOR¿
..
..
OH
O
..
..
..
..
C
Methan
oic acid
(form
ic acid)
H
O
O
..
..
..
..
C
Phenyl methan
oate
(phenyl form
ate)
H
OH
O
..
..
..
..
C
Ethan
oic acid
(acet
ic acid)
H
3
CH
3
C OCH
3
O
O
..
..
C
Methyl ethan
oate
(methyl acet
ate)
OH
O
..
..
..
..
C
Propan
oic acid
(propion
ic acid)
CH
3
CH
2
O
..
..
..
..
C
Ethyl propan
oate
(ethyl propionate)
CH
3
CH
2
OH
O
..
..
..
..
C
Butan
oic acid
(butyric acid)
CH
3
CH
2
CH
2
CH
3
CH
2
CH
2
O
O
..
..
..
..
C
tert-Butyl butan
oate
(tert-butyl butyrate)
OH
O
..
..
..
..
C
3-Methylpentano
ic acid
CH
3
CH
2
CHCH
2
CH
3
O
..
..
..
..
C
Ethyl 3-methylpentanoate
O
2
1
3
CH
3
CH
2
CHCH
2
CH
3
23
1
becomes
becomes
becomes
becomes
becomes
CH
2
CH
3
C(CH
3
)
3
CH
2
CH
3
WEB 3D
WEB 3D WEB 3D
FIGURE 18.3 Some examples of the
naming protocol for esters. For each
example, the R′ group in the drawing
and in the name are highlighted.
880 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
..
..
..
..
O
..
O
O
..
..
O
..
..
..
..
..
O
O
2-Oxacyclopentanone 2-Oxacycloheptanone
O
O
..
..
..
..
α -Lactone
γ -Lactone
β -Lactone
δ -Lactone
O
O
..
..
O
O
..
..
..
..
..
..
α
α
α
α
β
β
βδ
γ
γ
WEB 3D
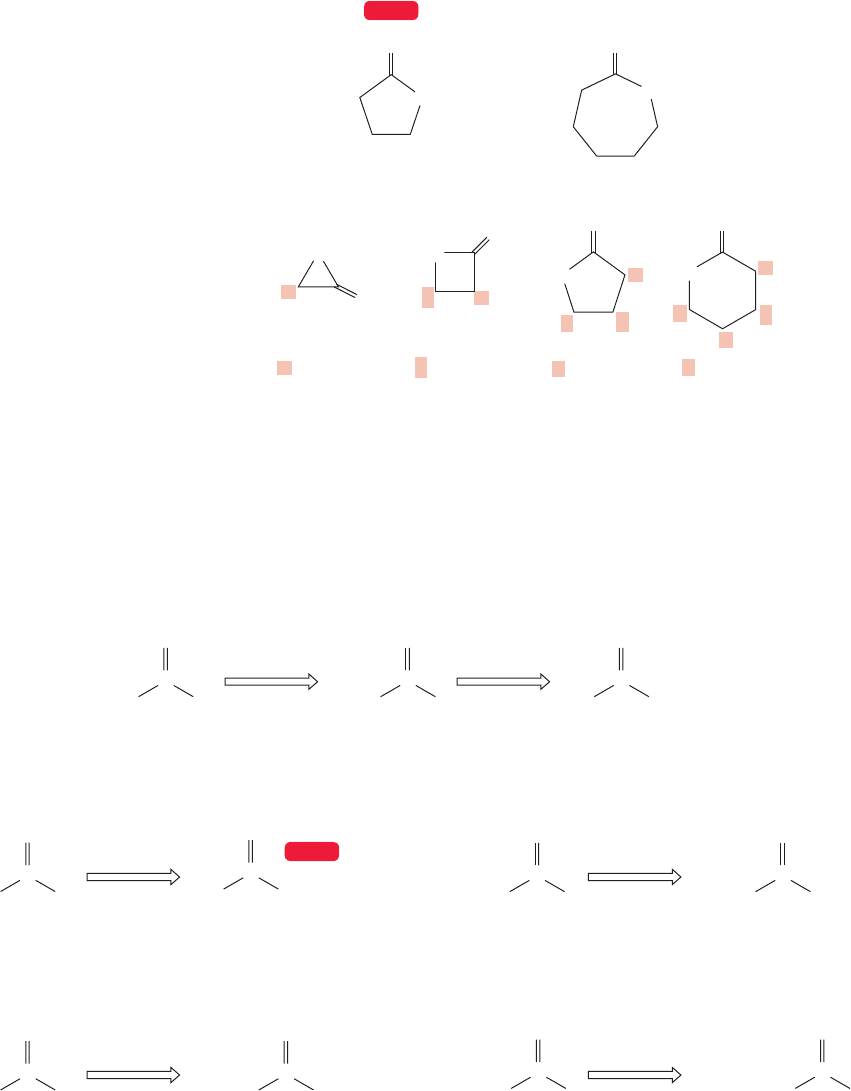
FIGURE 18.4 Cyclic esters are known
as lactones or oxacycloalkanones.
As we learned in Chapter 17 (p. 849), cyclic esters are called lactones. The IUPAC
system names these compounds as “oxa”cycloalkanones (Fig.18.4).Alternatively,the ring
size of a lactone can be described by starting at the carbonyl carbon and designating the
other carbons in the ring with Greek letters until the oxygen atom is reached. A three-
membered ring is an α-lactone, a four-membered ring is a β-lactone, and so on.
Cl
..
..
..
..
..
Cl
..
..
..
Cl
..
..
..
..
..
..
OH
O
..
..
C
Methanoic acid
(formic acid )
H
O
..
..
C
Methanoyl chloride
(formyl chloride)
Phosgene
H
ClCl
..
..
..
..
..
..
O
..
..
C
OH
O
..
..
..
..
C
Ethanoic acid
(acetic acid )
H
3
C
H
3
C
O
..
..
C
Ethanoyl chloride
(acetyl chloride)
OH
O
..
..
..
..
C
Propanoic acid
(propionic acid )
CH
3
CH
2
O
..
..
C
Propanoyl bromide
(propionyl bromide)
CH
3
CH
2
OH
O
..
..
..
..
C
Butanoic acid
(butyric acid )
CH
3
CH
2
CH
2
CH
3
CH
2
CH
2
O
..
..
C
Butanoyl fluoride
(butyryl fluoride)
OH
O
..
..
..
..
C
Pentanoic acid
CH
3
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
O
..
..
C
Pentanoyl chloride
Br
..
..
..
F
becomes
becomes
becomesbecomes
becomes becomes
WEB 3D
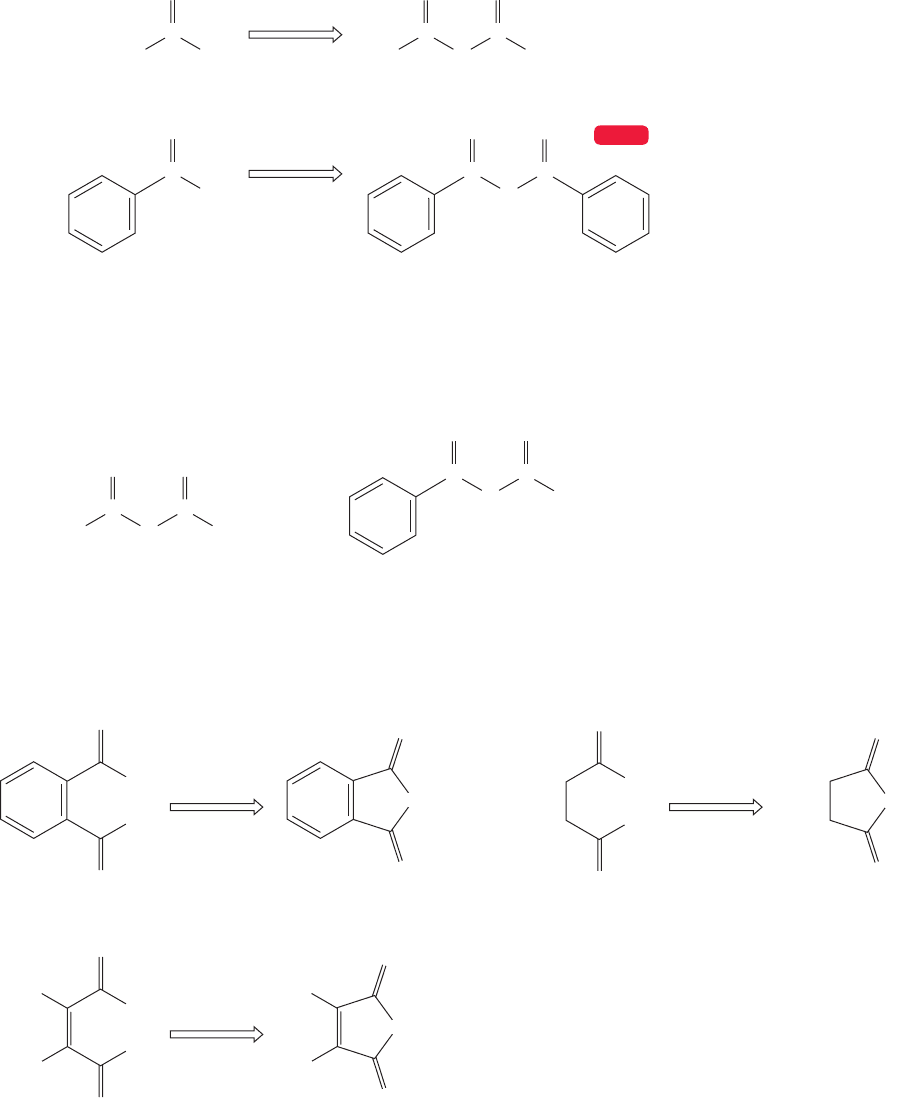
FIGURE 18.5 Examples of
the systematic and common
naming protocols for acid
halides.
Acid halides are named systematically by replacing the final ic acid of the car-
boxylic acid with yl halide.In practice, the names for the smaller acid halides are based
upon the common names for the related acids. Thus, acetyl chloride and formyl
fluoride would be understood everywhere. Formyl chloride is most unstable and
efforts to make it under most conditions are rewarded only with the formation of
HCl and CO. The diacid chloride of carbonic acid ( ) is known,
however, and this deadly poison is called phosgene (Fig. 18.5). Throughout this
Cl
O
CO
O
Cl
18.2 Nomenclature 881
OH
O
..
..
..
..
C
Ethanoic acid
(acetic acid)
Ethanoic anhydride
(acetic anhydride)
H
3
C O
O
..
..
..
..
C
O
..
..
C
H
3
CCH
3
OH
O
..
..
..
..
C
2
2
Benzoic acid Benzoic anhydride
O
O
..
..
..
..
C
O
..
..
C
become
become
WEB 3D
FIGURE 18.6 Examples of the
naming protocol for symmetrical
anhydrides.
chapter we will use acid chlorides as the example for acid halides. The other acid
halides are less stable, less accessible, and less studied.
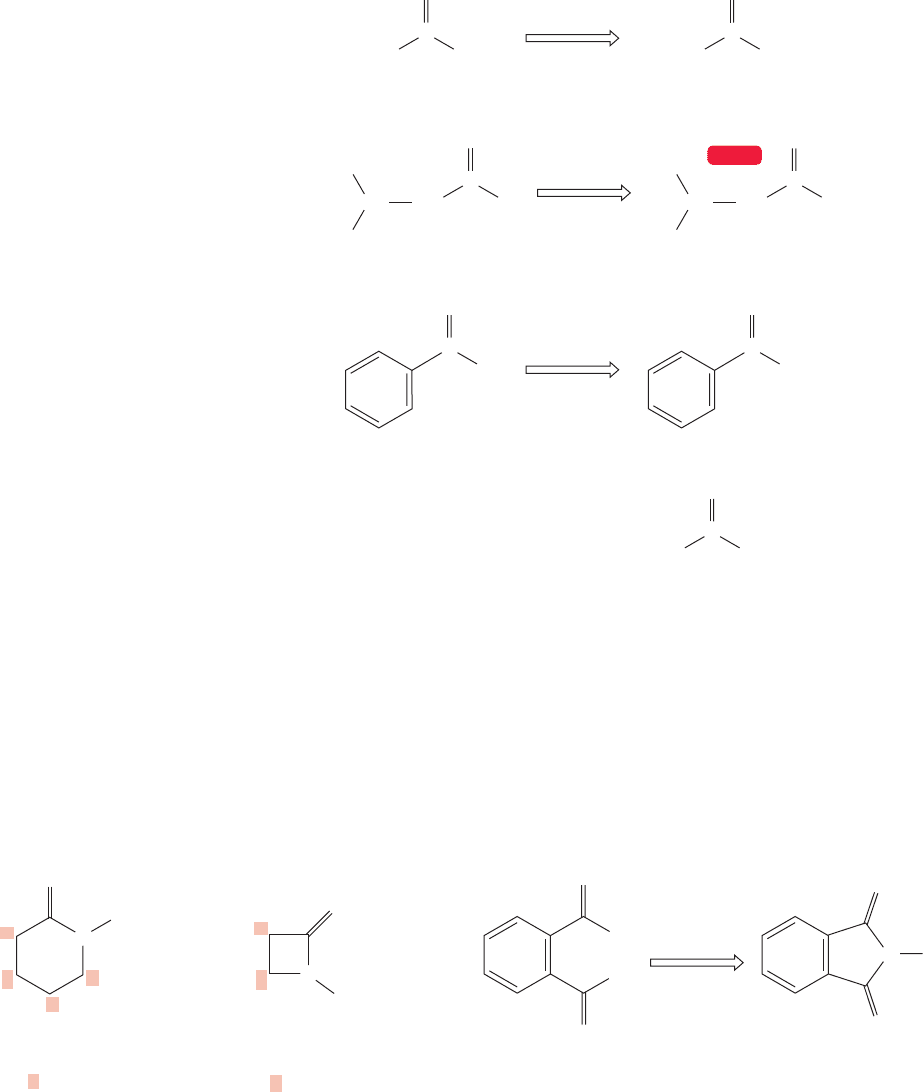
Acid anhydrides are named by reference to the carboxylic acid from which they
are formally derived by loss of water. The acid in the name is replaced by anhy-
dride. Both systematic and common names are used in this process (Fig. 18.6).
Mixed anhydrides present more problems. Here, both constituent acids must be
named and they are listed in alphabetical order.Two examples appear in Figure 18.7.
Ethanoic propanoic anhydride
(acetic propionic anhydride)
O
O
..
..
..
..
C
O
..
..
C
H
3
CCH
2
CH
3
CH
2
CH
2
CH
3
Benzoic butanoic anhydride
(benzoic butyric anhydride)
O
O
..
..
..
..
C
O
..
..
C
FIGURE 18.7 Named unsymmetrical
anhydrides.
Phthalic acid
Phthalic anhydride
OH
..
..
OH
..
..
O
..
..
O
..
..
O
..
..
O
..
O
..
..
..
H
H
Maleic acid
Maleic anhydride
OH
..
..
OH
..
..
H
H
O
..
..
O
O
..
..
Succinic acid
Succinic anhydride
OH
..
..
OH
..
..
O
..
..
O
O
..
..
..
..
O
..
O
..
..
..
..
..
O
..
O
..
..
..
becomes becomes
becomes
FIGURE 18.8 Examples of naming cyclic anhydrides.
Cyclic anhydrides are named as derivatives of the related carboxylic acids (Fig.18.8).
882 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
Amides, the nitrogen counterparts of esters, are systematically named by drop-
ping the final oic acid of the corresponding carboxylic acid and adding the suffix amide.
Substitution on nitrogen is indicated with a one-word prefix N-alkyl, as in N-methyl,
N,N-diethyl,and so on (Fig.18.9).Thus,an amide that has one alkyl group on the nitro-
gen is named as N-alkylalkanamide. An amide with two hydrogens on the nitrogen is
called a primary amide.A secondary amide has only one hydrogen on the amide nitrogen.
OH
O
..
..
..
..
C
Ethanoic acid
(acetic acid )
H
3
C
H
3
C
H
3
C
O
..
..
..
C
Ethanamide
(acetamide)
H
3
CNH
2
OH
O
..
..
..
..
C
3-Methylbutanoic acid
CH
2
CH
H
3
C
H
3
C
CH
2
CH
O
..
..
..
C
3-Methylbutanamide
NH
2
CH
3
CH
2
O
..
..
..
C
N,N-Diethylpropanamide
N(CH
2
CH
3
)
2
OH
O
..
..
..
..
C
Benzoic acid
O
..
..
..
C
Benzamide
NH
2
2
3
1
2
3
44
1
becomes
becomes
becomes
WEB 3D
FIGURE 18.9 Examples of the
naming protocol for amides.
A tertiary amide has no hydrogens on the nitrogen. Substituents on the nitrogen are
listed alphabetically together with the other substituents from the carboxylic acid por-
tion of the chain.As usual, the smaller members of the class have retained their com-
mon names, which are based on the common names for the smaller carboxylic acids.
For example, you will often encounter N,N-dimethylformamide (abbreviated DMF)
because it is a useful polar, high-boiling solvent.
Cyclic amides are known as lactams (p. 851), and the naming protocol closely
follows that for lactones,the cyclic esters.Lactams are named systematically as aza-
cycloalkanones. Imides are the nitrogen counterparts of anhydrides (Fig. 18.10).
Phthalic acid Phthalimide
OH
..
..
OH
..
..
O
..
..
NH
..
O
..
..
O
..
..
O
..
..
H
H
N
O
..
..
..
2-Azacyclohexanone
(δ-lactam)
2-Azacyclobutanone
(β-lactam)
..
..
O
N
α
α
β
βδ
γ
..
becomes
FIGURE 18.10 Cyclic amides are called lactams, and imides are the nitrogen counterparts of anhydrides.
18.2 Nomenclature 883
Compounds containing carbon–nitrogen triple bonds with the general formula
of RCN are named as nitriles. Systematically, these compounds are alkanenitriles.
Notice that the final “e” of the alkane root word is not dropped. The root word is
determined by counting the number of carbons in the longest chain, including the
carbon of the nitrile (Fig. 18.11). If the nitrile group is not the highest priority
group, the CN substituent is identified as a cyano- and listed as a prefix. Small
nitriles are commonly named as derivatives of the parent carboxylic acid or as
cyanides. Ethanenitrile, for example, is almost always called acetonitrile. It is a
common solvent.
OH
O
..
..
..
..
..
..
CCN
Ethanoic acid
(acetic acid)
Ethanenitrile
(acetonitrile, or
methyl cyanide)
H
3
C
H
3
C
OH
O
..
..
..
..
CCN
Propanoic acid
(propionic acid)
Propanenitrile
(propionitrile, or
ethyl cyanide)
CH
3
CH
2
CH
3
CH
2
becomes becomes
WEB 3D
FIGURE 18.11 A few examples of the naming protocol for nitriles.
PROBLEM 18.1 Draw structures for the following compounds: cyclopropyl
2-methylbutanoate, 3-chlorobutanoyl chloride, benzoic propanoic anhydride,
N,N-diethyl-4-phenylpentanamide, and ethylpropylketene.
..
..
C
H
H
C
Ethenone
(ketene)
O
..
..
C
H
H
3
C
C
1-Propen-1-one
(methylketene)
O
..
..
C
H
3
C
H
3
C
C
2-Methyl-1-propen-1-one
(dimethylketene)
O
..
..
C
H
3
C
CH
3
CH
2
C
2-Methyl-1-buten-1-one
(ethylmethylketene)
O
H
2
CC
Recall the allenes
CH
2
OC
and CO
2
O
..
..
C
H
C
2-Phenylethen-1-one
(phenylketene)
O
FIGURE 18.12 Ketenes are named as alkenyl ketones or as derivatives of the parent
unsubstituted ketene.
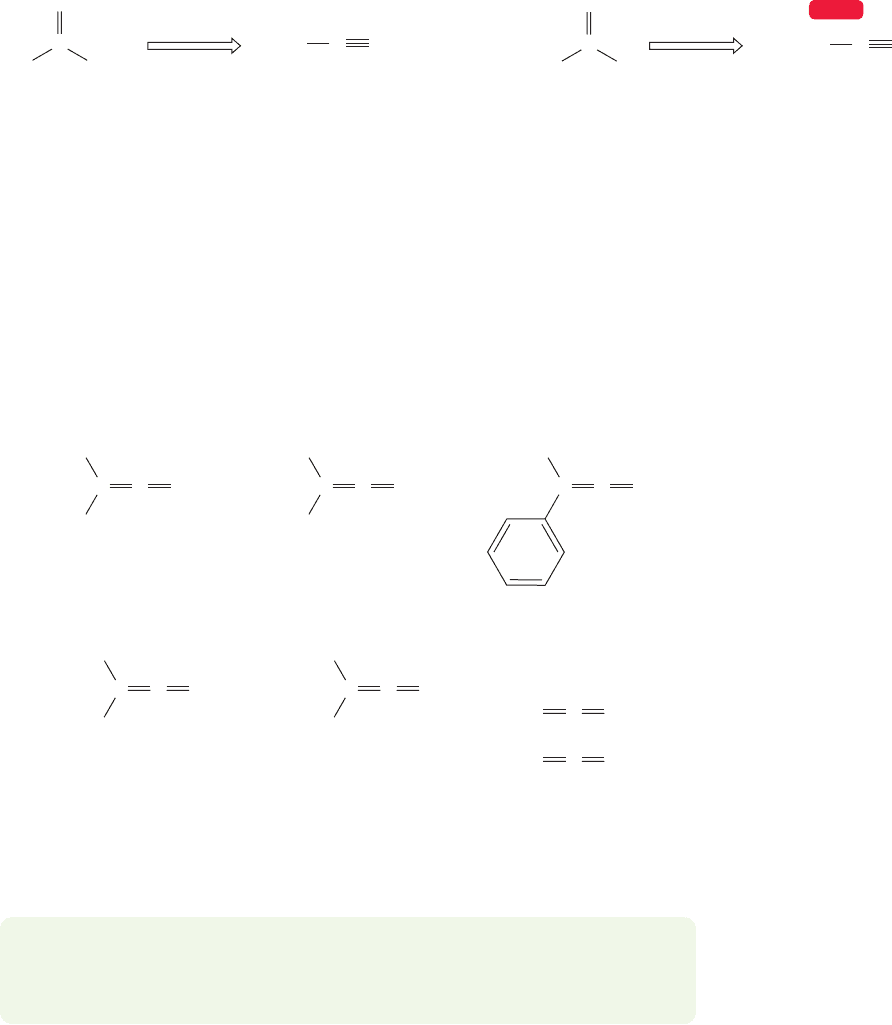
Ketenes (p. 515) are named using the IUPAC system as alkene-substituted
ketones (Fig. 18.12). Common names for ketenes are often used and are obtained
by simply describing the substituents on the ketene. Ketenes are related to allenes,
compounds with two directly attached carbon–carbon double bonds (Fig. 12.3,
p. 513).
884 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
TABLE 18.1 Some Properties of Acyl Compounds Related to Acetic Acid
Formula Common Name bp (°C) mp (°C)
CH
3
COOH Acetic acid 117.9 16.6
CH
3
COOCH
3
Methyl acetate 57 98.1
CH
3
COOCH
2
CH
3
Ethyl acetate 77 83.6
CH
3
COCl Acetyl chloride 50.9 112
CH
3
COBr Acetyl bromide 76 98
Acetic anhydride 139.6 73.1
CH
3
CONH
2
Acetamide 221.2 82.3
CH
3
CN Acetonitrile 81.6 45.7
Ketene 56 151H
2
C
P
C
P
O
CH
3
CO
O
O
O
COCH
3
18.3 Physical Properties and Structures
of Acyl Compounds
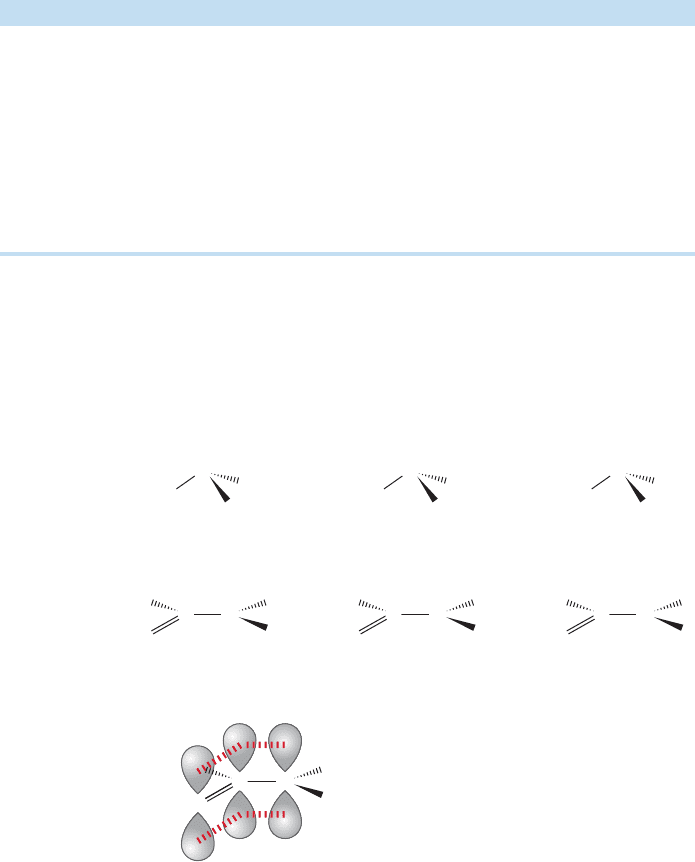
Acyl compounds are quite polar and have boiling points substantially higher than
those of the alkanes. Amides, like carboxylic acids, form hydrogen-bonded dimers
and oligomers, and are exceptionally high boiling. Table 18.1 gives some physical
properties of the acyl compounds related to acetic acid.
Pyramidal:
Planar:
Optimal overlap
requires planarity
Primary amine
Primary amide
N
H
H
R
..
N
HR
O
H
..
Secondary amine
Secondary amide
N
H
R
R
..
N
RR
O
H
..
Tertiary amine
Tertiary amide
N
R
R
R
..
N
RR
O
R
..
C
R
O
C
N
H
H
C
CC
FIGURE 18.13 Amides are stabilized
by resonance.
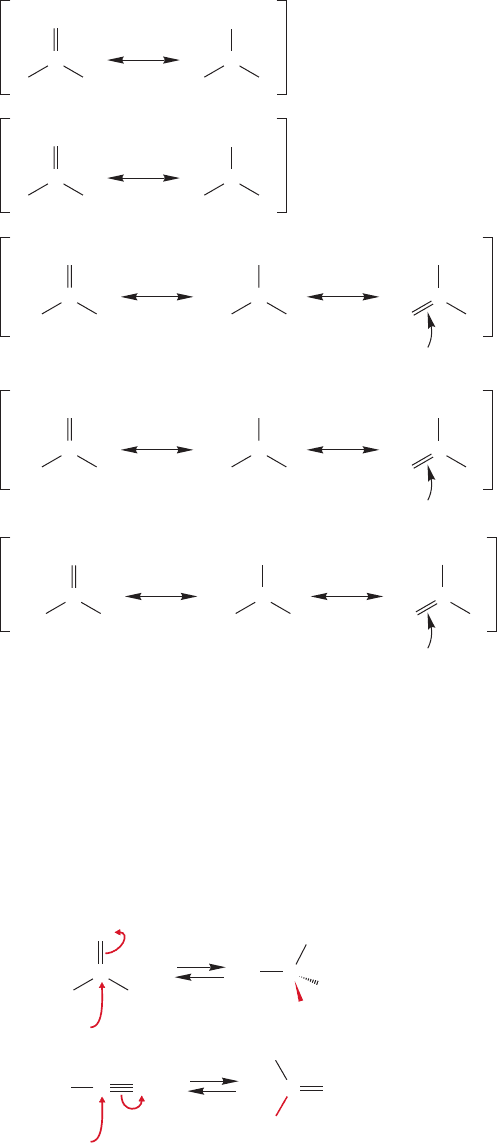
Resonance stabilization in these derivatives of carboxylic acids (acyl compounds)
requires maximum overlap between the carbonyl π orbital and the 2p orbital contain-
ing a pair of nonbonding electrons on the adjacent atom. This overlap has important
consequences for both structure and reactivity. So, for example, although amines are
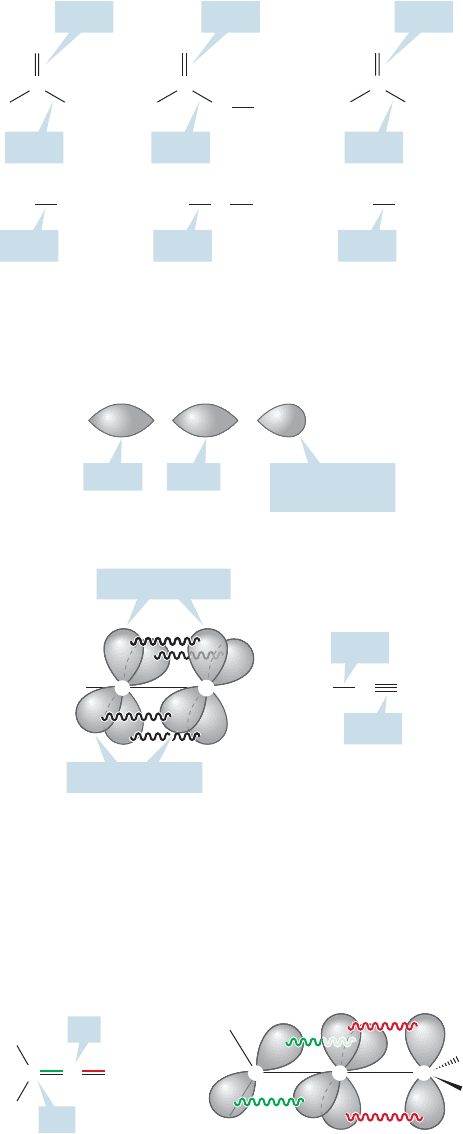
pyramidal,amides are flat.Figure 18.13 shows the resonance stabilization of amides.The
substantial stabilization conferred by resonance also makes amides less basic than amines.
The amount of resonance stabilization varies in these derivatives of carboxylic
acids.The acid halides are the least stabilized, followed by anhydrides, the esters and
acids, and finally amides, which are the most stabilized. Now two questions arise:
How do we know the relative amount of resonance stabilization, and what factors
contribute to the order? The first question must wait until the next section on spec-
troscopy, but we can deal with the second question right here.
First of all, the positive charge in the polar resonance form is better accom-
modated by the relatively electropositive nitrogen of amides than by the more
electronegative oxygen of esters and acids, or the chlorine of acid chlorides.
18.4 Acidity and Basicity of Acyl Compounds 885
Therefore, the polar resonance form contributes most in amides, and they are the
most stabilized. Second, in acid chlorides, resonance stabilization requires overlap
between a 3p orbital on chlorine and the carbonyl π orbital made up of 2p orbitals.
In the other acid derivatives the overlap is between 2p orbitals.
So,amides are the most stabilized, acid chlorides the least stabilized, and the other
acid derivatives come in between. Figure 18.14 shows the resonance forms for these
compounds as well as for the familiar aldehydes and ketones.
Esters
Amides
3p/2p Overlap
2p/2p Overlap
2p/2p Overlap
–
..
..
..
+
RO
..
..
ROR
O
..
..
C
R
O
..
..
C
–
..
+
..
RO R
O
..
..
C
Acid
chlorides
–
..
..
..
..
..
+
Cl
..
..
Cl
..
..
ClR
O
..
..
C
R
O
..
..
C
–
..
+
R
O
..
..
C
–
..
..
+
H
2
N
..
H
2
NR
O
..
..
C
R
O
..
..
C
–
..
+
H
2
N R
O
..
..
C
–
..
+
R¿ R
O
..
..
C
Ketones
R¿ R
O
..
..
C
–
..
+
HR
O
..
..
C
Aldehydes
HR
O
..
..
C
FIGURE 18.14 The acid derivatives
are stabilized by resonance, but the
extent of stabilization varies.
18.4 Acidity and Basicity of Acyl Compounds
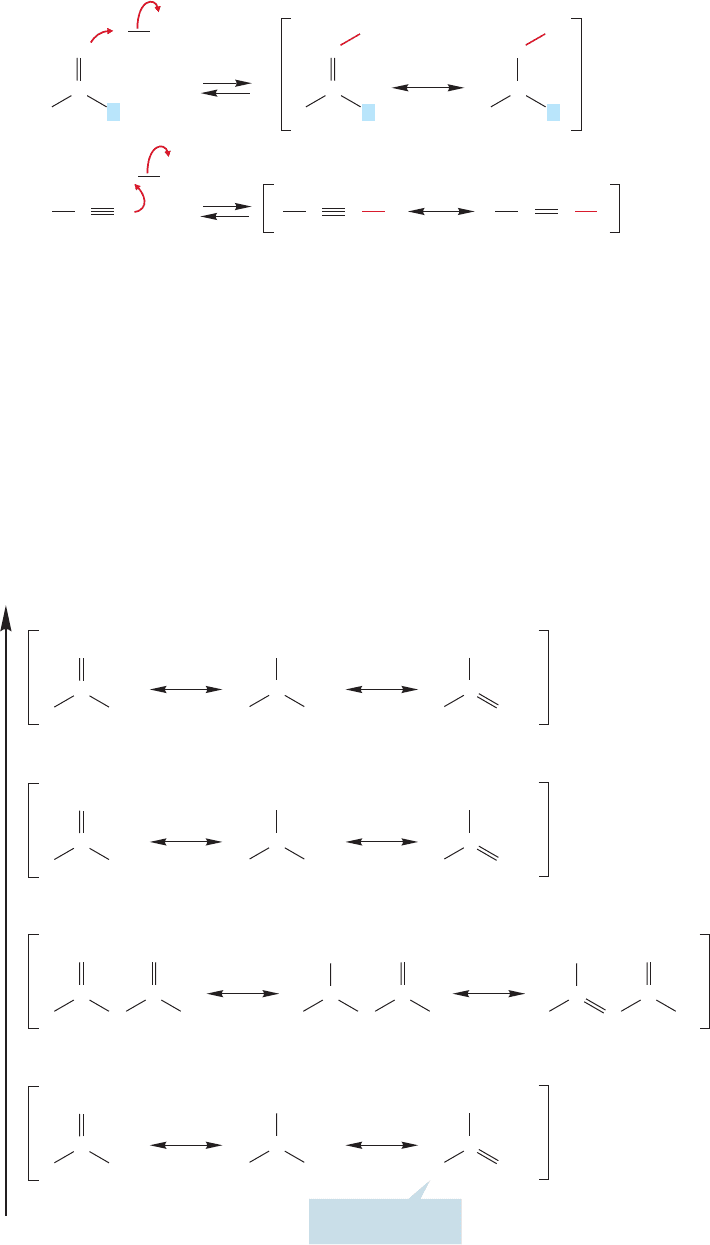
Acyl compounds lack the hydroxyl group of carboxylic acids and so are not strong
Brønsted acids. However, they are good Lewis acids—they act as electrophiles. The
carbonyl group is the source of this Lewis acidity. Nitriles, even though they have
no carbon–oxygen double bond, are also electrophiles.The electronegative nitrogen
strongly polarizes the carbon–nitrogen triple bond to the extent that nucleophiles
add to the nitrile carbon just like they add to carbonyl carbons (Fig. 18.15).
Nu
–
..
..
..
ROR
..
..
OR
O
..
..
O
..
..
C
CR
Nu
..
–
Nu
..
–
..
CRN
Nu
–
R
..
..
CN
FIGURE 18.15 The carbonyl group of
acyl compounds is the center of
Lewis acidity and can be attacked by
nucleophiles just as are the carbonyl
groups of aldehydes and ketones. An
analogous reaction occurs with
nitriles.
886 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
..
RL
O
..
..
C
R
H
L
O
..
C
+
R
H
L
O
..
..
..
COH
2
+
..
..
OH
2
+
OH
2
..
..
+
+
+
H
OH
2
..
+
H
..
CRN
C HRN
+
C HR
N
FIGURE 18.16 As these protonation
reactions show, acyl compounds and
nitriles can act as Brønsted bases.
Acyl compounds and nitriles are Brønsted bases and Lewis bases as well, with
the carbonyl oxygen or the nitrile nitrogen being the center of basicity (Fig.18.16).
Poor (high-energy)
resonance form
Stronger Lewis base
More charge on oxygen
More resonance stabilization
..
RNH
2
..
RNH
2
NH
2
O
..
..
C
..
..
OR
..
..
OR
..
OR
–
..
+
+
O
..
..
C
–
..
R
O
..
..
C
RR
O
..
..
C
–
..
+
+
O
..
..
C
–
..
R
O
..
..
C
..
..
RCl R
O
..
..
..
..
Cl
..
..
Cl
..
..
C
–
..
+
+
O
..
..
C
–
..
R
O
..
..
C
Amide
Ester
..
..
OR
O
..
..
C
R
O
..
..
C
..
..
OR
O
..
..
C
R
O
..
..
C
+
–
..
..
OR
O
..
..
C
R
O
..
..
C
+
–
..
Anhydride
Acid chloride
FIGURE 18.17 The carbonyl
oxygens of amides are stronger
nucleophiles than are those of
esters. Acid chloride carbonyls
are the weakest nucleophiles of all.
As we have seen,all these acid derivatives are stabilized by dipolar resonance forms
in which the L group bears a positive charge and the carbonyl oxygen a negative
charge (Fig. 18.14). Because the acid chlorides are the least stabilized by this res-
onance,they have the least basic carbonyl oxygen atoms. Esters and carboxylic acids
are stabilized by resonance to a similar extent and therefore are roughly equally
basic. In amides it is a nitrogen atom, not an oxygen atom, that bears the positive
charge. As was mentioned earlier, nitrogen is less electronegative than oxygen and
therefore bears this positive charge with more grace than oxygen. The result is a
more polar carbonyl group for the amide and a more basic carbonyl oxygen atom
(Fig. 18.17).
18.5 Spectral Characteristics 887
18.5 Spectral Characteristics
18.5a Infrared Spectra Characteristic strong carbonyl stretching frequen-
cies are observed for all these compounds (except, of course, for the nitriles, which
contain no carbonyl group). The contribution of the dipolar resonance forms
can be seen in the position of the stretch. Ketenes have a very strong car-
bonyl bond, as the inner carbon is hybridized sp, not the usual sp
2
(Fig. 18.20).
C
P
O
1.43 A
⬚
1.78 A
⬚
1.33 A
⬚
1.20 A
⬚
1.78 A
⬚
1.19 A
⬚
..
..
OCH
3
..
..
O
H
O
..
..
C
..
..
H
3
CCl
O
..
..
..
..
Cl
..
..
C
H
H
3
C
1.47 A
⬚
1.35 A
⬚
1.22 A
⬚
..
HNH
2
..
NH
2
O
..
..
C
H
3
C H
3
C
FIGURE 18.18 The structures of
some simple acyl compounds. Notice
how the carbon–oxygen and
carbon–nitrogen single bonds are
shortened by the double-bond
character that results from the
resonance shown in Figure 18.17.
CCH
3
CH
3
C
sp
3
/sp
2p
y
/2p
y
Overlap
sp/sp
Two electrons
in an sp orbital
C
σ System
π System
H
3
CN
..
..
..
..
.
.
.
.
1.16 A
⬚
1.46 A
⬚
..
NN
2p
z
/2p
z
Overlap
FIGURE 18.19 The σ and π orbital
systems of acetonitrile and the bond
lengths.
..
C
H
H
H
H
C
The green and red
π
bonds are not as alike
as it seems at first
C
.
..
.
..
O
sp
2
sp
..
..
..
..
O C
=
FIGURE 18.20 The bonding scheme
in ketene.
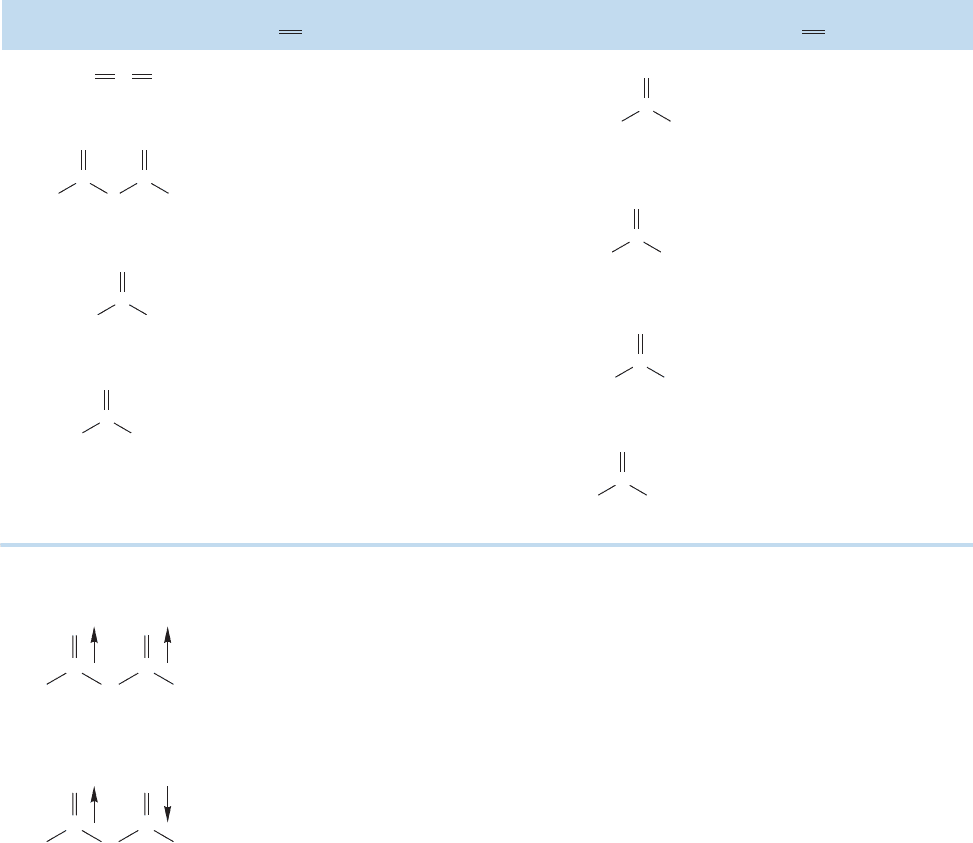
Resonance stabilization influences the bond lengths in these compounds as well
(Fig. 18.18). Although the carbon–chlorine bond distance is little changed in acid
chlorides from that in alkyl chlorides,the carbon–oxygen and carbon–nitrogen bond
distances in esters and amides are shortened compared to alcohols and amines,
reflecting some double-bond character in these compounds.
Nitriles are linear molecules with short carbon–nitrogen bond distances. The sp
hybridization of the carbon and nitrogen atoms ensures this. Figure 18.19 gives orbital
pictures of both the σ and π systems,which closely resemble those of the alkynes (p.125).
888 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
The more s character in a bond, the stronger it is. In addition, the ketene carbonyl
has no substituent to feed electrons into its π system. Therefore, ketenes absorb in
the IR at very high frequency ( 2150 cm
1
).
The acid chloride carbonyl is the strongest of the acid derivatives,with the most
double-bond character, so its stretch appears at the highest frequency.
Anhydrides, esters, and carboxylic acids are next. Amides, in which the contribu-
tion from the dipolar resonance form is strongest, and the single-bond character
of the carbonyl group is greatest, have the lowest carbonyl stretching frequencies
(Table 18.2).
C
P
O
'
TABLE 18.2 Infrared Stretching Frequencies of Some Carbonyl Compounds
H
2
C OC
H
3
C
C
O
O
C
O
CH
3
H
3
C
C
O
Cl
H
3
C
C
O
OCH
3
Compound
Ketene
Acetic anhydride
Acetyl chloride
Methyl acetate
H
3
C
C
O
H
H
3
C
C
O
NHCH
3
H
3
C
C
O
OH
H
3
C
C
O
CH
3
Compound
Acetaldehyde
Acetone
Acetic acid
N-Methylacetamide
2151
1833, 1767
1799
1750
Frequency of
C O in CCl
4
(cm
–1
)
1733
1719
1717
1688
Frequency of
C O in CCl
4
(cm
–1
)
O
Coupled symmetrical stretch
O
..
..
..
..
C
O
..
..
C
RR
O
Coupled unsymmetrical stretch
O
..
..
..
..
C
O
..
..
C
RR
FIGURE 18.21 The two carbonyl
stretching frequencies of anhydrides.
In the anhydrides, which contain two carbonyl groups, there are usually two
carbonyl stretching frequencies. This doubling is not the result of independent
stretching of the separate carbonyls, because symmetrical anhydrides as well as
unsymmetrical anhydrides show the two IR bands. Instead, the two bands are
caused by the coupled symmetrical and unsymmetrical stretching modes of these
compounds (Fig. 18.21). Remember, vibrations of bonds in molecules are no
more independent of each other than are the vibrations of attached real springs
(p. 708).
Nitriles show a strong carbon–nitrogen triple-bond stretch at characteristically
high frequency (2200–2300 cm
1
). Notice that this is quite near the frequency of
the related carbon–carbon triple bonds (2100–2300 cm
1
).
18.5b Nuclear Magnetic Resonance Spectra Hydrogens in the α position
are deshielded by the carbonyl or nitrile group, and therefore appear at relatively low
field, δ 2.0–2.7 ppm in a
1
H NMR spectrum. The carbonyl carbons of acyl com-
pounds, and the nitrile carbons of cyanides bear partial positive charges and appear
