Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

18.6 Reactions of Acid Chlorides: Synthesis of Acyl Compounds 889
downfield in the
13
C NMR spectra (Table 18.3). Carbon NMR spectroscopy is a
useful tool for differentiating between a ketone (or aldehyde) and an acid derivative
because the carbonyl carbon of a ketone appears near δ 200 ppm and the acid deriv-
ative appears near δ 170 ppm.
PROBLEM SOLVING
The words “NMR spectrum” and a change in the spectrum with temperature
always mean that some groups in the molecule are interchanging positions
through some sort of molecular motion, typically bond rotation (as here) or ring
flipping in cyclohexanes.
..
H N(CH
3
)
2
O
..
..
C
N,N-Dimethylformamide
(DMF)
PROBLEM 18.2 In the room temperature
1
H NMR spectrum of N,N-dimethyl-
formamide (DMF), two methyl signals are observed. As the temperature is raised
they merge into a single signal. Explain.
18.6 Reactions of Acid Chlorides: Synthesis
of Acyl Compounds
As mentioned before, all acyl compounds participate in the addition–elimination
process. Acid chlorides are especially reactive toward nucleophiles.Their carbonyl
groups, being the least stabilized by resonance, have the highest energy and are
the most reactive. So, an initial addition reaction with a nucleophile is relatively
easy. The chloride atom of acid chlorides is an excellent leaving group, and
sits poised, ready to depart once the tetrahedral intermediate has been formed
H
␣
C
O
HH
3
C
H
␣
C
O
ClH
3
C
TABLE 18.3 Some NMR Properties of Carbonyl Compounds and CH
3
CN (ppm, CDCl
3
)
H
␣
C
O
CH
3
H
3
C
Compound
Acetaldehyde
Acetone
Acetyl chloride
Ethyl acetate
H
␣
C
O
OCH
2
CH
3
H
3
C
H
␣
CNH
3
C
H
␣
C
O
N(CH
3
)
2
H
3
C
Compound
N,N-Dimethylacetamide
Acetonitrile
199
205
169
13
C ␦
(C O, C N)
2.25
2.18
2.66
␦ (H
␣
)
169
170
117
13
C ␦
(C O, C N)
2.05
2.09
2.05
␦ (H
␣
)
Acid chloride aminolysis
890 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
SOME SPECIFIC EXAMPLES
C
Tetrahedral intermediate
–
–
–
Nu
Nu
Nu
..
–
–
–
..
..
R
R
Cl
O
..
..
..
..
..
Cl
..
..
O
..
..
C
..
..
R
Cl
O
..
..
..
..
C +
addition
of Nu
–
THE GENERIC ADDITION–
ELIMINATION REACTION
elimination
of chloride
Primary amide
Secondary amide
Tertiary amide
..
..
RCl
O
..
..
..
..
C
..
..
ROH
O
..
..
C
Acids
Esters
RN
3
N
3
O
..
..
C
Acyl azides
R
O
..
..
C
R
O
..
..
C
R
O
..
..
C
RCN
O
..
..
CN
..
..
..
C
Acyl nitriles
..
..
O
..
..
HOR
..
..
HOH
Anhydrides
R
O
..
..
C
..
..
O
R
O
..
..
..
C
R
O
..
..
C
..
R
R
H
N
..
R
R
N
O
..
..
C
GENERAL REACTIONS
..
NH
2
NH
3
..
NH
2
R
..
NHR
2
..
..
OR
pyridine
0 ⬚C
..
..
..
H
3
C
H
3
C
Cl
O
..
..
..
C
..
..
..
..
C
H
H
C
CH
3
CH
2
S
CH
3
CH
2
OH
Cl
O
..
..
..
..
..
C
H
3
C
..
..
CC
CH
3
CH
2
S
OCH
2
CH
3
O
..
..
..
..
C
H
3
C
O
..
..
C
+
NH
2
CH
3
H
3
CCH
3
..
NH
CH
3
Amide (80%)
Ester (100%)
..
..
H
2
O
H
H
..
..
CC
PhS
PhS
Cl
O
..
..
..
..
..
C
..
..
CC
PhS
PhS
OH
O
..
..
.. ..
..
.. ..
..
C
Acid (82%)
H
3
CCH
3
FIGURE 18.22 Addition–elimination
reactions of acid chlorides. Note the
synthetic potential.
(Fig. 18.22). The result is an exceedingly facile and common example of the
addition–elimination mechanism.
PROBLEM 18.3 Acid chlorides often smell like hydrochloric acid. Explain.
18.6 Reactions of Acid Chlorides: Synthesis of Acyl Compounds 891
Many nucleophiles are effective in the addition–elimination reaction of acid chlo-
rides, and a great many acyl compounds can be made using acid chlorides as start-
ing materials.
Of course,strong nucleophiles such as organolithium compounds,Grignard reagents,
and the metal hydrides also react rapidly with acid chlorides. The problem here is one
of controlling secondary reactions.For example,reduction of an acid chloride with lithi-
um aluminum hydride initially gives an aldehyde. But the newly born aldehyde finds
itself in the presence of a reducing agent easily strong enough to reduce it further to the
alkoxide, and hence to the primary alcohol after water is added (Fig. 18.23).
THE GENERAL CASE
A SPECIFIC EXAMPLE
The aldehyde is born
in the presence of LiAlH
4
and must react further
Final product
primary alcohol
..
..
H
2
O
C
Li H AlH
3
H
H
+
Li
+
–
–
..
..
R
R
Cl
O
..
..
..
..
..
Cl
..
..
O
..
..
C
–
RH
..
O
H
..
..
–
..
..
OH
+
..
CH
2
..
OH
R
..
C
R
O
..
–
..
..
Cl
..
..
..
C
addition
LiAlH
4
addition
again
elimination
protonation
C
C
..
..
Cl
O
..
..
..
..
Cl
..
..
O
..
..
CH
2
OH
(95%)
..
..
CH
2
OH
..
..
1. LiAlH
4
ether
2. H
3
O /H
2
O
+
FIGURE 18.23 The reaction of an
acid chloride with lithium aluminum
hydride leads to a primary alcohol.
The hydride adds twice.
Similarly, reaction of an acid chloride with an organometallic reagent, ,
initially gives a ketone that usually reacts with a second equivalent of to give
the tertiary alcohol (Fig. 18.24). Note that this reaction must give a tertiary alcohol
in which at least two R groups are the same.
R
O
M
R
O
M
THE GENERAL CASE
A SPECIFIC EXAMPLE
..
..
H
2
O
This ketone is born in the presence of an
organolithium reagent and must react further
C
C
LiR
R
R
R
R
+
Li
+
–
–
..
..
R
R
Cl
O
..
..
..
..
..
Cl
..
..
..
..
Cl
O
..
..
..
C
CH
2
CH
3
CH
2
CH
3
OH
..
(93%)
O
..
..
CR
..
OH
..
C
R
O
..
–
..
..
..
Cl
..
..
..
C
addition
1. 2 equiv.
CH
3
CH
2
MgBr
ether
2. H
3
O /H
2
O
+
1. RLi
2.
elimination
FIGURE 18.24 The reaction of an
acid chloride with an organometallic
reagent proceeds all the way to the
tertiary alcohol.The organometallic
reagent adds twice.
892 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
All is fine if we are trying to make these particular alcohols, but often we are not.
How to stop the process at the aldehyde or ketone stage is the problem, and several
solutions have been found over the years. Less reactive organometallic reagents or
metal hydrides allow the isolation of the intermediate aldehydes or ketones.The reac-
tivity of lithium aluminum hydride can be attenuated by replacing some of the hydro-
gens with other groups.For example,use of lithium aluminum tri-tert-butoxyhydride
allows isolation of the aldehyde (Fig. 18.25). Presumably, the bulky metal hydride is
unable to reduce the aldehyde, which is less reactive than the original acid chloride.
THE GENERAL CASE
A SPECIFIC EXAMPLE
Lithium aluminum
tri-tert-butoxyhydride
C
H
H
..
..
R
R
Cl
O
..
..
..
..
Cl
..
..
..
..
..
Cl
O
..
..
(85%)
C
R
O
..
–
..
C
Stable under
these conditions
+
addition
1. LiAl[OC(CH
3
)
3
]
3
H
–78 ⬚C
2. H
2
O
elimination
Li H Al[OC(CH
3
)
3
]
3
+
–
C
NO
2
..
H
O
..
C
NO
2
Cl
..
..
..
..
Li
+
–
..
O
..
..
FIGURE 18.25 Lithium aluminum
tri-tert-butoxyhydride is not reactive
enough to add to the initially formed
aldehyde.
Catalytic reduction of an acid chloride using a deactivated, or “poisoned” cata-
lyst is called the Rosenmund reduction after Karl W. Rosenmund (1884–1964)
(Fig. 18.26). In this reaction, only the more reactive acid chloride is reduced.
THE GENERAL CASE
A SPECIFIC EXAMPLE
H
..
..
RCl
O
..
..
..
C
R
O
..
..
C
H
2
/Pd
Quinoline
a catalyst poison
quinoline
145 ⬚C
C
..
..
Cl
O
..
..
..
C
(77%)
H
O
..
..
H
2
/ BaSO
4
/Pd
, S
..
N
FIGURE 18.26 The Rosenmund
reduction of an acid chloride gives
an aldehyde.
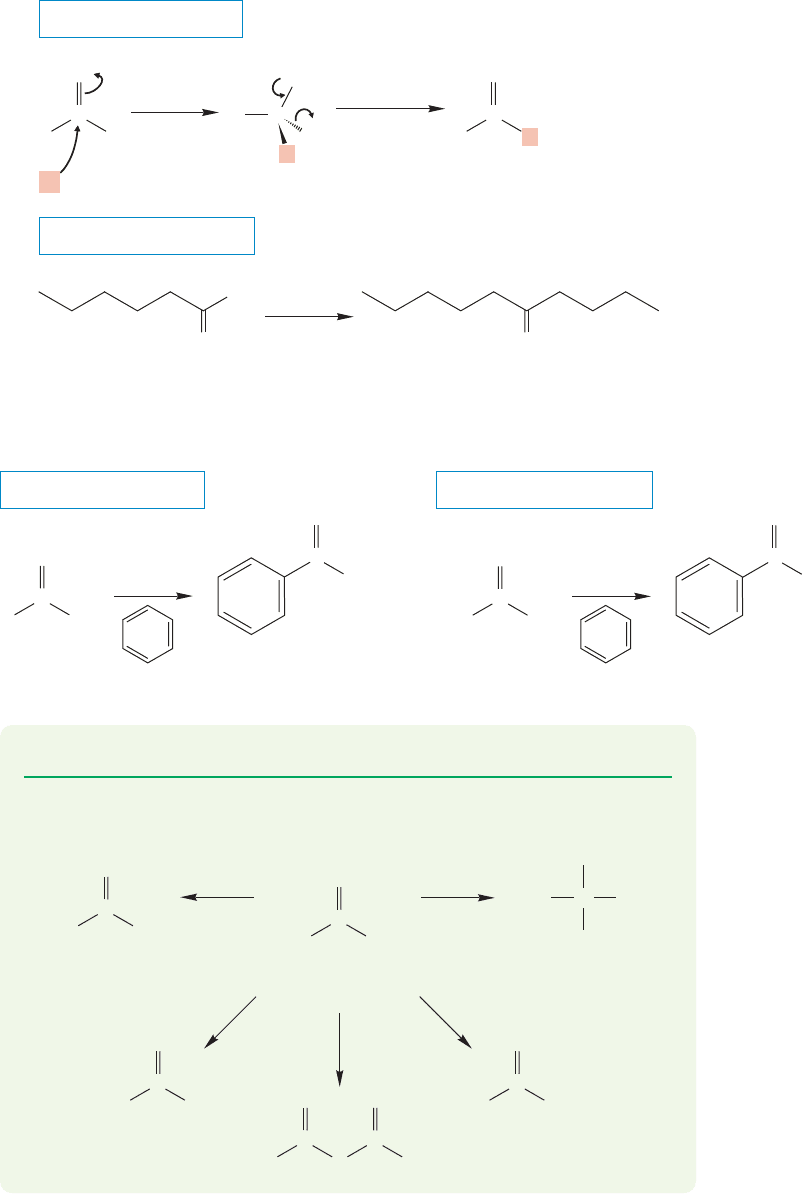
18.6 Reactions of Acid Chlorides: Synthesis of Acyl Compounds 893
The more stable, less reactive aldehyde can be isolated. Although this is not the same
poisoned catalyst used for hydrogenation of an alkyne to give the cis alkene (Lindlar
catalyst, p. 452), they are very similar.
Organocuprates react with acid chlorides but are not reactive enough to add to
the product ketones (Fig. 18.27).
THE GENERAL CASE
A SPECIFIC EXAMPLE
C
Li
+
..
..
R
R
Cl
O
..
..
..
..
Cl
..
..
..
Cl
..
..
..
..
C
R
O
..
–
..
..
Cl
..
..
..
C
addition
Bu
2
CuLi
elimination
LiR
2
Cu
R
R
+
–
Li
+
–
..
O
..
..
O O
(79%)
..
..
FIGURE 18.27 Cuprates are reactive
enough to add R
to the carbonyl
group of the acid chloride, but not
reactive enough to attack the product
ketones.
THE GENERAL CASE
A SPECIFIC EXAMPLE
..
..
RCl
O
..
..
..
C
R
O
..
..
C
AlCl
3
..
..
H
3
CCl
O
..
..
..
C
CH
3
O
..
..
C
(97%)
AlCl
3
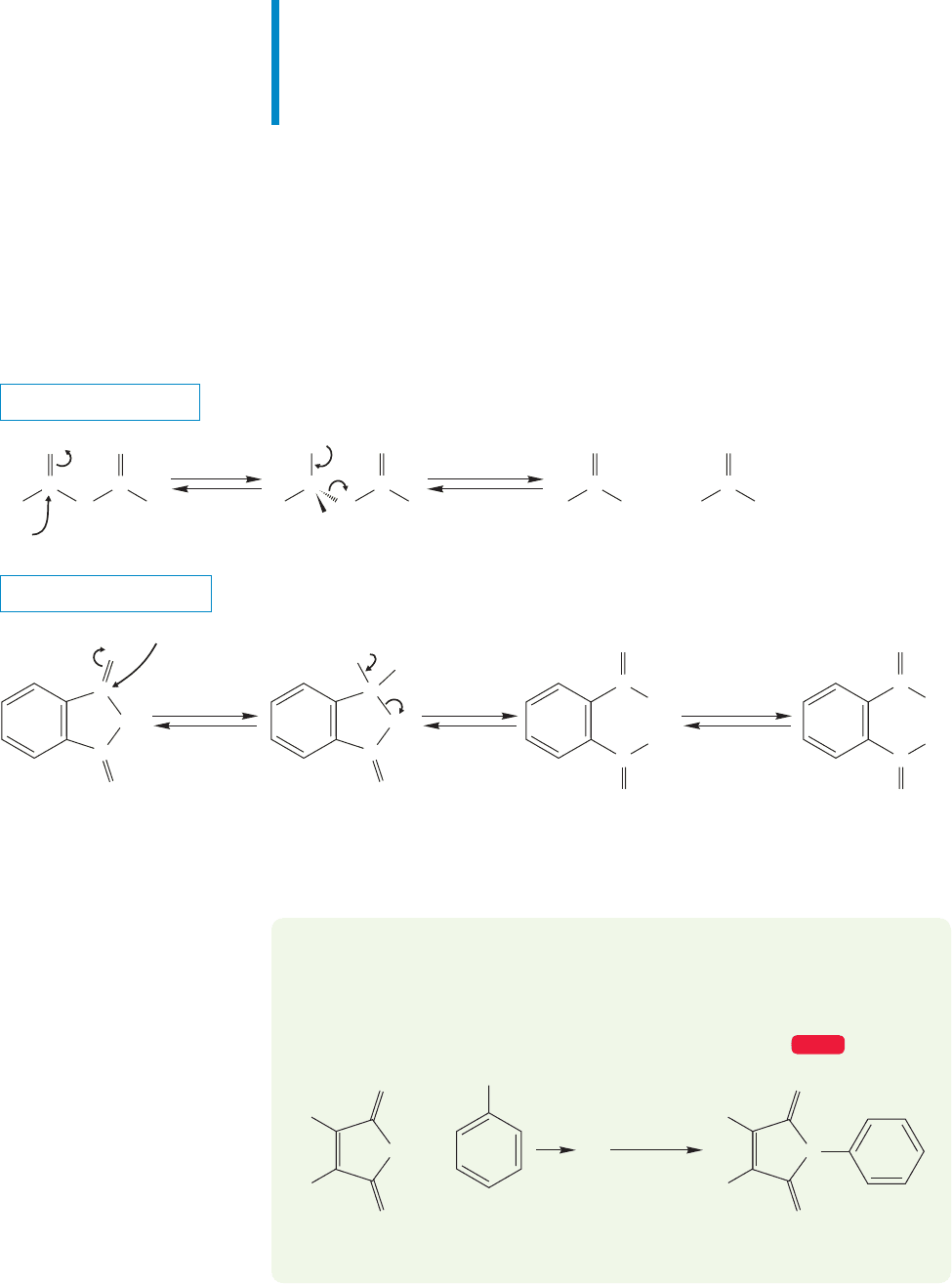
FIGURE 18.28 Friedel–Crafts acylation of benzene produces an aromatic ketone.
Finally, don’t forget that acid chlorides are used in the synthesis of aromatic ketones
through Friedel–Crafts acylation (p.643).This very useful reaction is shown in Figure 18.28.
PROBLEM 18.4 Write a mechanism for the formation of the product of Figure 18.28.
PROBLEM 18.5 Starting with acetyl chloride, devise a synthesis of each of the
following molecules.
H
3
CCl
O
C
Acetyl chloride
H
3
C OCH
3
O
C
(a)
H
3
COH
O
C
H
3
C
O
C
(b)
H
3
CO
O
C
CH
3
O
C
(c)
(e)
H
3
CCH
3
CH
3
OH
CH
2
CH
3
C
(d)
894 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
Summary
The presence of the good leaving group (chloride) attached directly to the
carbon–oxygen double bond makes all manner of addition–elimination reactions
possible for acid chlorides. The acid chloride can be used to make anhydrides,
esters, carboxylic acids, amides, aldehydes, ketones, and alcohols.
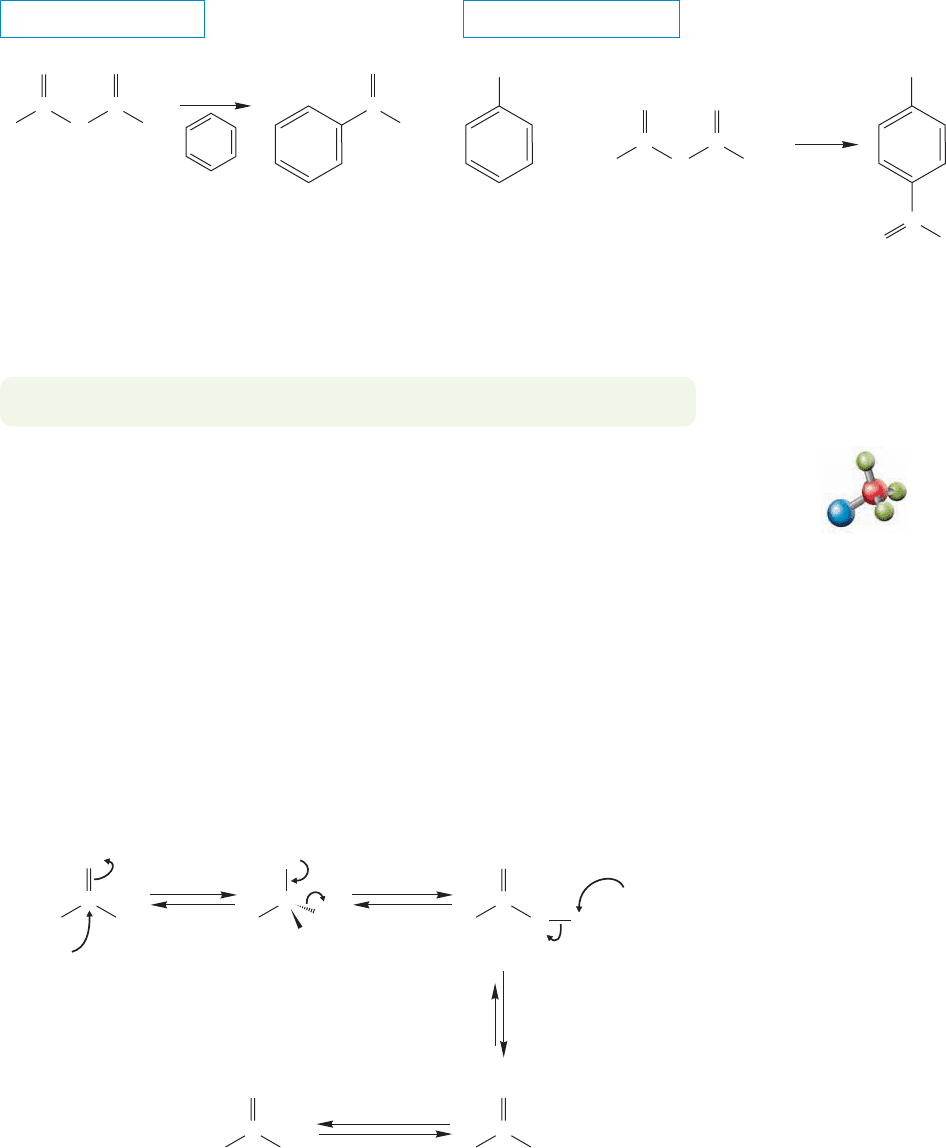
18.7 Reactions of Anhydrides
The reactivity of anhydrides is similar to that of acid chlorides. A carboxylate anion
is the leaving group in a variety of syntheses of acyl derivatives,all of which are exam-
ples of the addition–elimination process (Fig. 18.29). One reaction of this kind is
the basic hydrolysis of phthalic anhydride to phthalic acid. Here the leaving group
is an internal carboxylate anion.
THE GENERAL CASE
A SPECIFIC EXAMPLE
+
K
+
OH
Carboxylate
leaving group
..
H
3
O
..
+
/H
2
O
Nu
Nu
Nu
..
–
C
C
Phthalic anhydride Phthalic acid
O
O
..
..
..
..
..
..
..
..
C
O
..
..
O
..
..
..
..
..
O
..
..
O
..
C
R
O
..
..
C
RRO
..
..
O
..
..
..
C
R
O
O
..
..
..
..
..
C
O
..
..
C
RR
–
–
OH
..
..
..
–
–
..
O
..
..
..
..
..
–
–
..
addition
addition
elimination
protonation
elimination
O
C
C
O
OH
C
C
O
..
..
O
..
..
..
..
OH
..
..
O
C
C
O
..
..
O
..
..
..
..
OH
..
..
OH
..
/H
2
O
..
..
FIGURE 18.29 Addition–elimination reactions of an anhydride.
PROBLEM 18.6 Provide a mechanism for the formation of N-phenylmaleimide
from maleic anhydride and aniline. Be sure you give a structure for the interme-
diate A, and account for the role of the acetic anhydride in the second step.
NH
2
Maleic
anhydride
O
..
..
..
O
..
..
O
..
..
O
..
..
..
..
O
A
Aniline
acetic
anhydride
+
H
H
..
N
N -Phenylmaleimide
H
H
WEB 3D
18.8 Reactions of Esters 895
Like acid chlorides, anhydrides can be used in the Friedel–Crafts reaction
(Fig. 18.30). Once again, a strong Lewis acid catalyst such as aluminum chloride is
used to activate the anhydride. The mechanism follows the pattern of the Friedel–
Crafts reactions we saw earlier (p. 643).
PROBLEM 18.7 Write a mechanism for the reaction of Figure 18.30.
THE GENERAL CASE
A SPECIFIC EXAMPLE
+
O
O
..
..
..
..
C
O
..
..
C
AlCl
3
AlCl
3
RR
O
..
..
C
C
(96%)
R
O
O
O
..
..
..
..
..
C
O
..
..
..
C
H
3
CCH
3
CH
3
CH
3
CH
3
FIGURE 18.30 A Friedel–Crafts reaction using an anhydride as the source of the acyl group.
18.8 Reactions of Esters
Esters are less reactive than acid chlorides and anhydrides in addition reactions, but
more reactive than amides. Esters can be converted into their parent carboxylic acids
under either basic or acidic aqueous conditions in a process called, logically enough,
ester hydrolysis. In base, the mechanism is the familiar addition–elimination one
(Fig. 18.31). Hydroxide ion attacks the carbonyl group to form a tetrahedral
intermediate. Loss of alkoxide then gives the acid, which is rapidly deproto-
nated to the carboxylate anion in basic solution. Notice that this reaction, saponi-
fication (p. 862), is not catalytic.The hydroxide ion used up in the reaction is not
regenerated at the end. To get the carboxylic acid itself, a final acidification step
is necessary.
..
..
H
3
O
..
+
/H
2
O
..
..
H
2
O +
HO
..
–
O
..
..
..
..
OH
..
..
..
..
C
R
O
..
..
..
C
R
O
..
..
..
..
C
Tetrahedral
intermediate
R
–
–
..
addition
elimination
acidification
OR
OR
..
..
OR
..
..
ROH
..
–
deprotonation of
the carboxylic acid
..
..
O
O
..
..
C
R
..
..
O
+
O
..
..
C
R
..
..
OH
H
FIGURE 18.31 The base-induced
ester hydrolysis reaction,
saponification.
Ester hydrolysis
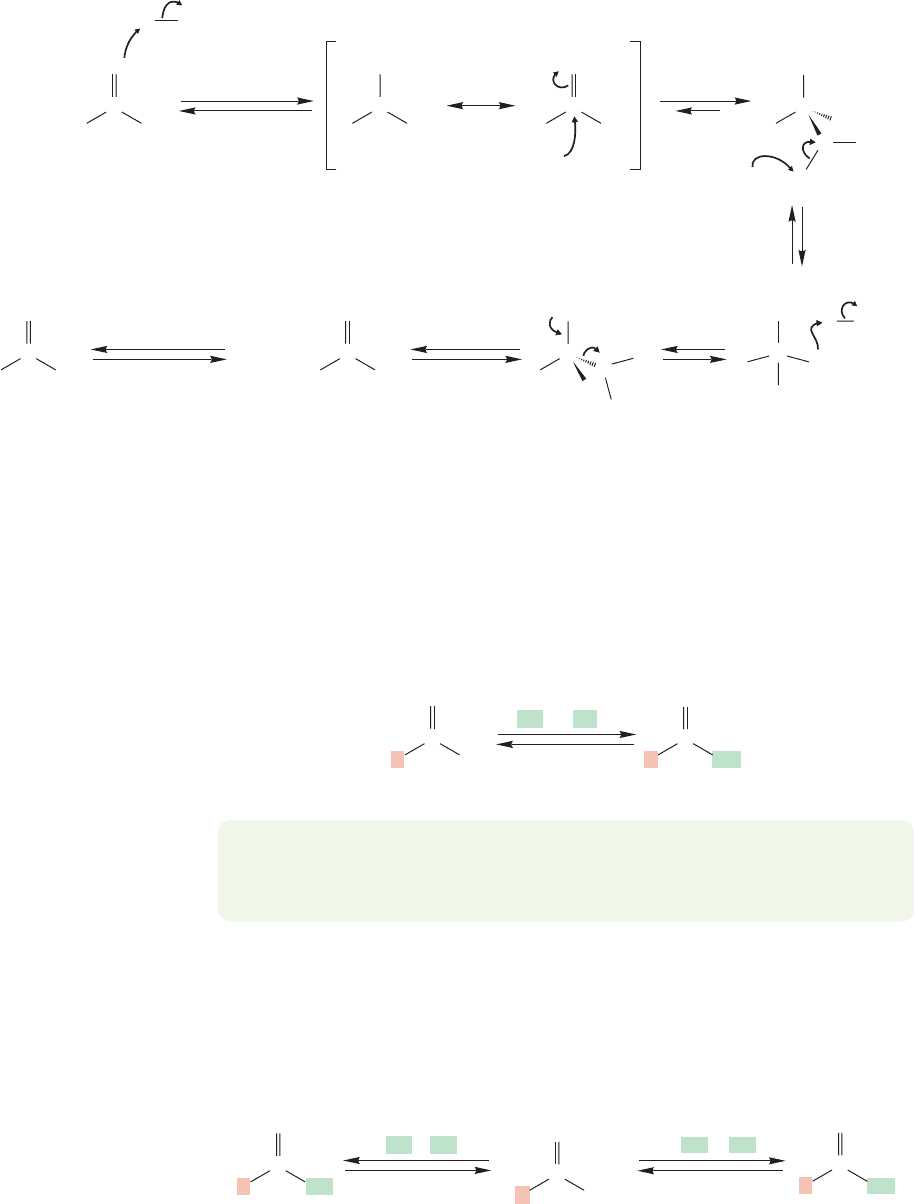
896 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
Esters can also be converted into carboxylic acids in acid (Fig. 18.32). The
carbonyl oxygen is first protonated to give a resonance-stabilized cation to which
water adds. Water is by no means as nucleophilic as hydroxide, but the protonated
carbonyl is a very strong electrophile and the overall reaction is favorable. The
reaction concludes with proton transfers and loss of alcohol.
H
3
O
..
+
+
+
..
..
H
2
O
..
..
H
2
O
+
OH
..
..
O
..
..
C
R
OH
..
..
..
C
R
..
OH
..
..
OH
..
..
addition
elimination protonation
deprotonation
..
..
..
H
3
O
+
+
/H
2
O
protonation
OR
OH
..
..
..
C
R
+
OH
+
OH
..
..
..
..
C
R
+
OR
..
HOH
2
H
H
O
..
..
..
..
C
ROR
O
..
..
..
..
C
ROH
..
..
C
ROR
OR
HO
..
OH
..
..
..
C
R
R
..
H
O
..
..
ROH+
OH
2
..
+
H
deprotonation
FIGURE 18.32 The acid-catalyzed ester hydrolysis reaction.
Have you seen this reaction before? You certainly have. It is the exact reverse of
the mechanism for Fischer esterification (p. 841), so there is nothing new here at all.
What determines where the overall equilibrium settles out? The structure of the ester
is an important factor, but much more important are the reaction conditions. Excess
water favors the acid; excess alcohol favors the ester. Le Châtelier’s principle is at
work here, and the chemist has the ability to manipulate this equilibrium (Fig. 18.33)
to favor either side. In practice, most acids and esters are easily interconvertible.
Fischer
esterification
ester hydrolysis
..
..
H
3
O
..
+
+
/H
2
O
..
..
C
ROR
O
..
..
..
..
O
..
..
C
ROH
..
.. ..
ROH
2
/ ROH
FIGURE 18.33 Acids and esters are
usually interconvertible.
PROBLEM 18.8 Where appropriate, draw resonance forms for the intermediate
species in the reactions of Figure 18.33.You will have to write mechanisms for the
reactions first.
A reaction closely related to acid-catalyzed ester hydrolysis is acid-catalyzed
transesterification. In this reaction, an ester is treated with an excess of an alcohol
and an acid catalyst.The result is replacement of the ester OR group with the alco-
hol OR group (Fig. 18.34). Like ester hydrolysis, transesterification can be carried
out under either acidic or basic conditions.The mechanisms are extensions of those
you have already seen in the ester hydrolysis reactions or Fischer esterification.
..
..
C
ROR
O
..
..
..
..
C
ROR
O
..
..
–
..
.. ..
.. ..
ORHOR /
base catalyzed
..
..
C
ROR
O
..
..
+
acid catalyzed
..
.. ..
H
2
OR / HOR
FIGURE 18.34 Base- and acid-
catalyzed transesterification.
18.8 Reactions of Esters 897
PROBLEM 18.9 Write mechanisms for acid- and base-induced transesterification.
WORKED PROBLEM 18.10 There is an important difference between the reaction
of an ester with hydroxide (
OH) and of an ester with alkoxide (
OR).The reac-
tion with hydroxide is neither catalytic nor reversible, whereas the reaction with
alkoxide is both catalytic and reversible. Analyze the mechanisms of these reac-
tions and explain these observations.
ANSWER Look at the final products of the two reactions. Reaction with hydroxide
leads to a carboxylic acid (pK
a
4.5) and an alkoxide ion. These two species
must react very rapidly to make the more stable carboxylate anion (resonance sta-
bilized) and the alcohol (pK
a
17). The hydroxide reagent is consumed in this
reaction, and the overall process is so thermodynamically favorable that it is irre-
versible in a practical sense.
'
'
O
..
..
–
..
..
..
C
OR
R
..
..
OR
..
..
O
..
..
C
R
..
O
..
..
C
OH
H
R
..
..
OH
–
(–)
–
+
..
..
..
OR
O
..
..
With an alkoxide reagent, things are different. Now the product is not a car-
boxylic acid but another ester. There is no proton that can be removed, and there
is a new molecule of alkoxide generated. The reaction is approximately ther-
moneutral. The reaction is reversible.
OR
+
O
..
..
C
OR
R
..
..
–
..
..
..
+
O
..
..
C
R
..
..
–
..
..
..
OR
OR
WORKED PROBLEM 18.11 Given that there are acid- and base-catalyzed transester-
ification reactions, should there not be both acid- and base-catalyzed Fischer
esterifications as well? Explain clearly why there is no base-catalyzed version of
Fischer esterification (i.e., RCOOH RO
RCOOR HO
does not occur).
ANSWER The reaction of a carboxylic acid with an alkoxide can’t proceed by
addition–elimination to give an ester because there is another much easier reac-
tion available; that reaction is simple removal of the carboxylic acid hydroxyl
proton to give the resonance-stabilized carboxylate anion. They don’t call these
compounds “acids” for nothing! The lesson in this problem is that you have to
“think simple.” Look first for “trivial” reactions (loss of the proton) before pro-
ceeding on to more complicated processes (addition–elimination).
U
OR
..
..
O
..
..
C
R
..
O
..
..
C
OH
H
R
..
..
–
–
..
..
..
OR
O
..
..
pK
a
~4.5 pK
a
~17
+
O
..
..
C
R
–
..
..
O
..
–
..
..
..
RO
–
..
..
..
HO
O
..
..
C
OR
R
..
..
O
..
..
OH
R
..
..
C
++
898 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
PROBLEM 18.12 Esters can also react with amines to give amides. Write a mecha-
nism for the reaction of methyl acetate (CH
3
COOCH
3
) and ammonia to form
acetamide (CH
3
CONH
2
).
Summary
Anhydrides react with water to generate carboxylic acids, with alcohols to give
esters, and with amines to form amides. Esters behave similarly. There are both
acid-catalyzed and base-induced reactions of esters with water (ester hydrolysis)
to give carboxylic acids. There are both acid- and base-catalyzed versions of the
reaction of esters with alcohols (transesterification) that generate new esters.
Esters also react with amines to form amides.
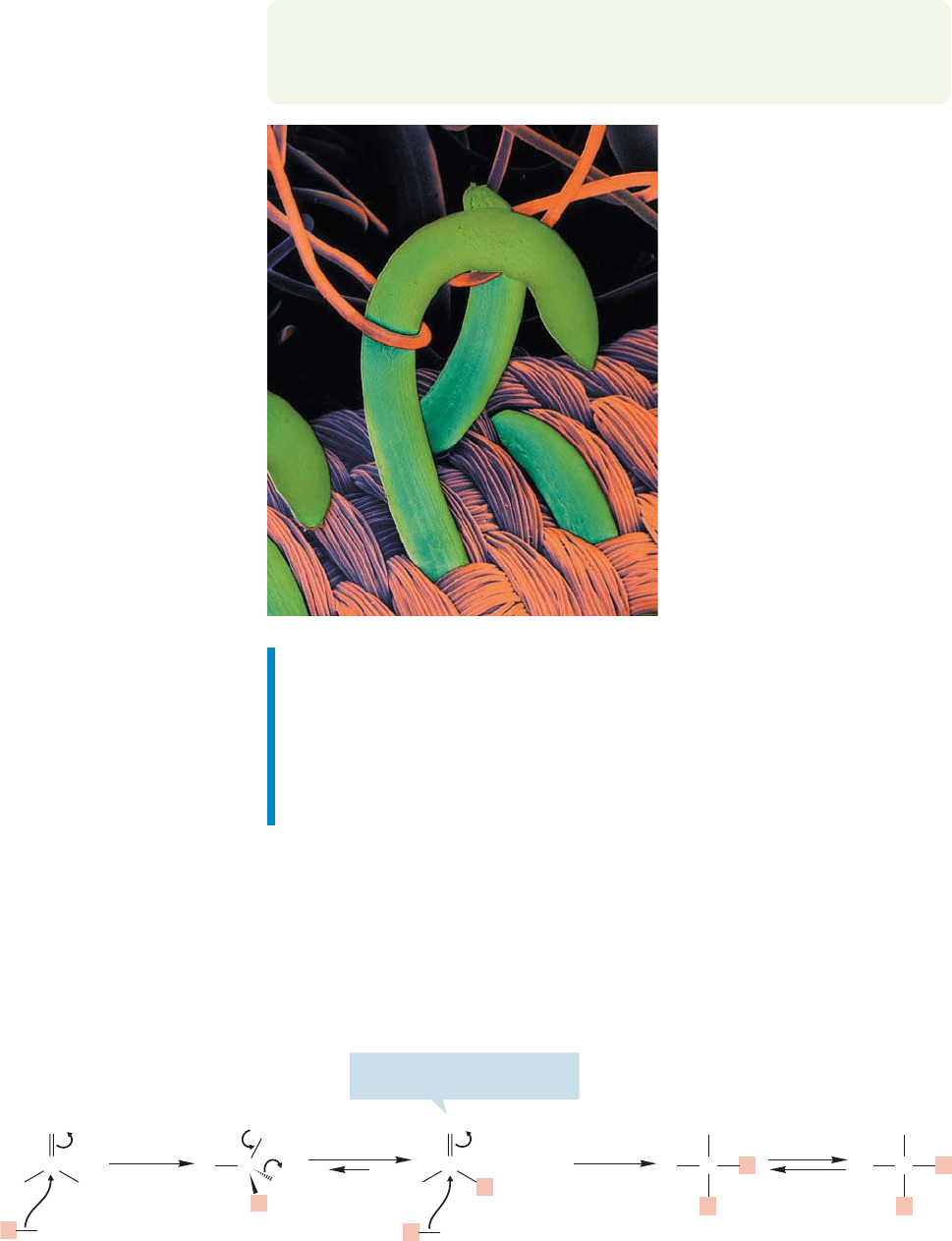
Esters react with amines to make
amides. One of the most important
synthetic polymers is nylon. It can
be made through the reaction of a
diester with a diamine.The
resulting polymer is a polyamide
(p. 852).This electron microscope
photo illustrates the binding
mechanism for Velcro, which is
a polyamide.
+
H
3
O
..
+
..
The relatively reactive ketone
cannot usually be isolated
C
Tetrahedral
intermediate
Alcohol
MgBrR
MgBrR
R
R
R
R
BrMg
+
..
..
R
R
OR
O
..
..
..
OR
..
..
..
C
–
–
R
..
O
..
..
R
R
CR
..
OH
..
C
R
O
..
..
C
first
addition
second
addition
elimination
BrMg
+
–
..
O
..
..
RO
FIGURE 18.35 The reaction of an ester with a Grignard reagent to form a tertiary alcohol.
Organometallic reagents and metal hydrides react with esters in still another
example of the addition–elimination process.Usually, these strong nucleophiles react
further with the ketones that are the initial products of the reactions (Fig. 18.35).
The ketones are not as well stabilized by resonance as are the esters and so are more
reactive in the addition reaction.
The reaction shown in Figure 18.35 is another general synthesis of complex
tertiary alcohols (compare with Fig. 18.24). Here are the possibilities for the
