Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

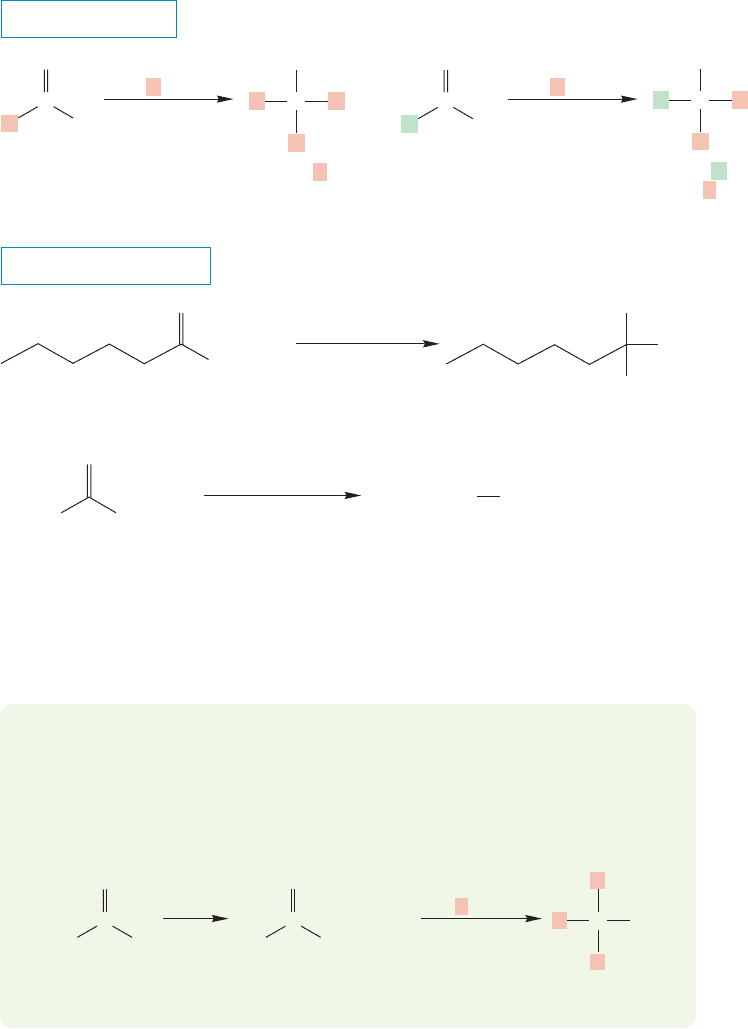
18.8 Reactions of Esters 899
addition of two equivalents of an organometallic reagent with an ester. First, ter-
tiary alcohols in which all three R groups are the same can be made if the R group
of the organometallic reagent is the same as that of the ester alkyl group. Second,
the R of the organometallic reagent can be different from that of the alkyl part
of the ester. In this case, the tertiary alcohol will contain two different R groups
(Fig. 18.36).
Because two R groups must come from the organometallic reagent, there is no
way to use this ester-based synthesis to make a tertiary alcohol with three different
R groups.
R
SPECIFIC EXAMPLES
O
..
..
CH
3
CH
3
..
OH
..
..
..
CH
3
CH
2
OCH
2
CH
3
..
..
OH
O
..
..
2. H
3
O /H
2
O
1. 2 equiv.
CH
3
MgBr
1. 2 equiv.
CH
3
CH
2
MgBr
OCH
2
CH
3
..
..
(CH
3
CH
2
)
3
C
(82%)
(83%)
..
..
..
+
2. H
3
O /H
2
O
..
..
..
+
GENERAL CASES
..
..
ROR
R
R
C
All three R’s
the same
R
..
OH
..
C
1. 2 RMgBr
..
..
ROR
R
R
C
One R
two R’s
R
..
OH
..
C
2. H
3
O /H
2
O
..
..
..
+
1. 2 RMgBr
2. H
3
O /H
2
O
..
..
..
+
O
..
..
O
..
..
FIGURE 18.36 Tertiary alcohols in which all three R groups are the same, or molecules in
which only two R groups are the same can be made, but the structural type in which all
three R groups are different cannot be made this way. Alkyllithium reagents, RLi, can also
be used.
+
..
..
..
2. H
2
O/ H
3
O
..
OR
..
RO
O
..
..
R
R
CR
..
..
OH
C HCl
Carbonates AlcoholsPhosgene
1. 3 RLi
..
..
..
Cl
..
Cl
O
..
..
..
..
..
..
C
ROH
......
..
..
+
PROBLEM 18.13 Dialkyl carbonates can be made from the reaction of phosgene
with alcohols. These carbonates are converted into tertiary alcohols with three
identical R groups on reaction with three equivalents of a Grignard reagent or an
organolithium reagent. Provide mechanisms for the formation of carbonates and
tertiary alcohols in these reactions.
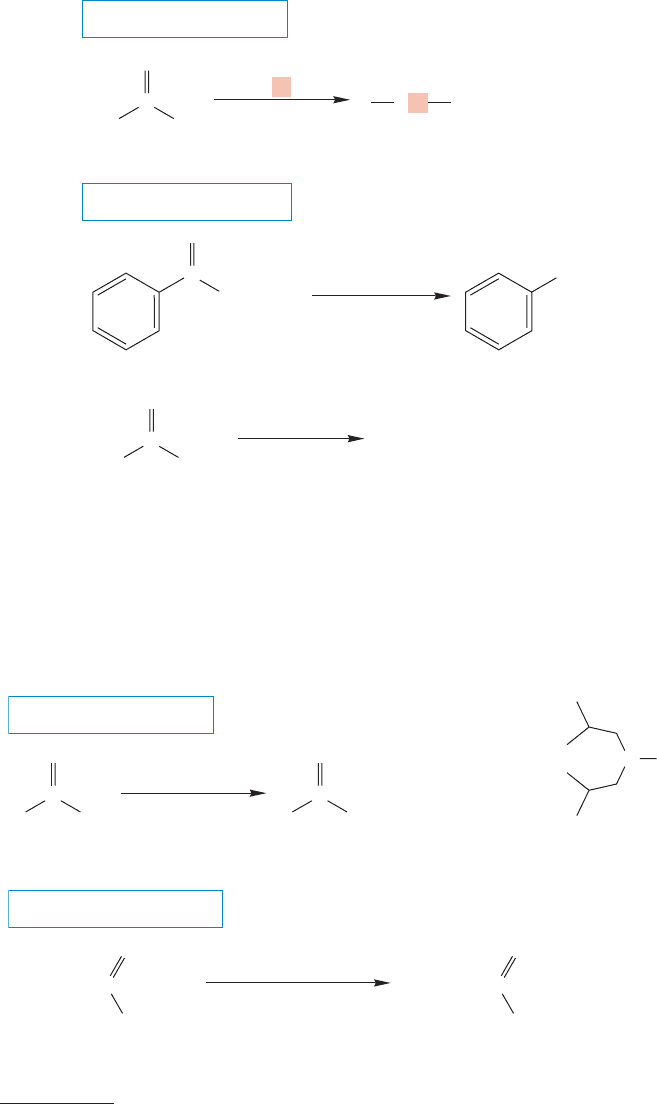
900 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
THE GENERAL CASE
SPECIFIC EXAMPLES
/H
3
O
..
+
..
..
ORR
CH
2
R
..
..
OH
C
..
..
..
..
OCH
2
CH
3
C
1. LiAlH
4
..
..
2. HCl/H
2
O
..
..
OCH
3
H
3
C
CH
3
CH
2
OH
..
..
C
1. LiBH
4
..
..
2. H
2
O
/H
3
O
..
+
..
..
2. H
2
O
1. LiAlH
4
CH
2
OH
(90%)
(90%)
O
..
..
O
..
..
O
..
..
FIGURE 18.37 The reduction of
esters with the powerful hydride
donor lithium aluminum hydride
leads to primary alkoxides and then,
after acidification, to primary
alcohols.
Metal hydride reduction of esters is difficult to stop at the intermediate alde-
hyde stage, and the usual result is further reduction to the primary alcohol
(Fig.18.37). Either LiAlH
4
or LiBH
4
(but not NaBH
4
,which will not reduce esters)
is the reagent of choice.
As with acid chlorides, other metal hydrides that permit isolation of the inter-
mediate aldehydes have been developed. For example, diisobutylaluminum hydride
(DIBAL-H) reduces ketones to alcohols, but can be used to prepare the interme-
diate aldehydes from esters (Fig. 18.38).
2
THE GENERAL CASE
A SPECIFIC EXAMPLE
Al
..
..
2. H
2
O
..
..
2. H
2
O
..
..
ORR
O
..
..
C
HR
O
..
..
C
1. DIBAL-H
..
..
OCH
2
CH
3
O
..
..
CH
3
(CH
2
)
10
C
..
H
O
..
CH
3
(CH
2
)
10
C
1. DIBAL-H, –70 ⬚C
hexane
DIBAL-H =
Diisobutylaluminum
hydride
H
FIGURE 18.38 If DIBAL-H is used
as the hydride donor, the reduction
of esters can often be stopped at
the aldehyde stage.
2
This reaction requires low temperature and is actually finicky. See Problem 18.54.
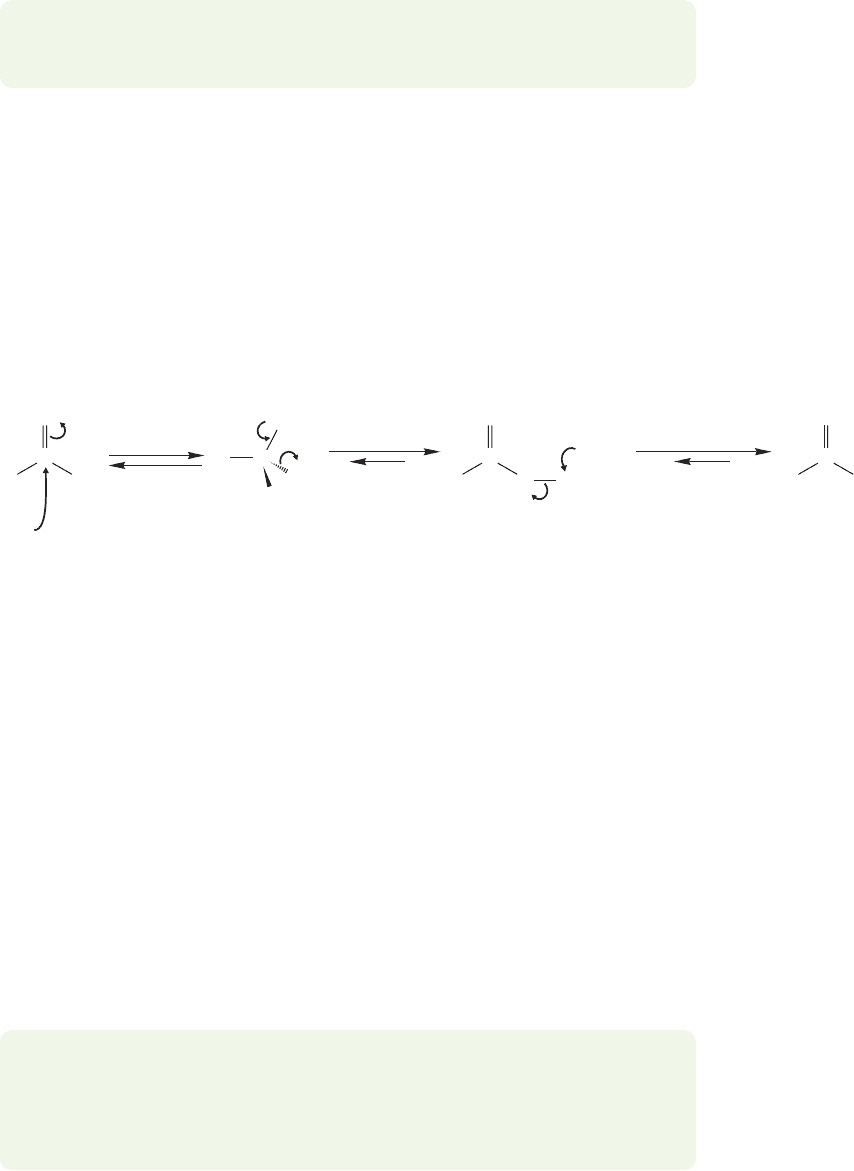
18.9 Reactions of Amides 901
18.9 Reactions of Amides
Even though amides are the least reactive of the acyl compounds, they can never-
theless be hydrolyzed in either acid or base. The mechanisms of these reactions are
directly related to those of acid- or base-induced ester hydrolysis. In base, the car-
bonyl carbon of an amide is attacked to give tetrahedral intermediate A (Fig. 18.39).
PROBLEM 18.14 Suggest one reason why DIBAL-H might be slower than
LiAlH
4
to react with the aldehyde of Figure 18.38.
Esters also have a substantial chemistry involving the related enolate anions. We
will develop this subject in detail in Chapter 19.
..
..
H
2
O
+C
A
–
–
(–)
–
..
R
R
NH
2
..
NH
2
..
NH
2
NH
3
Ammonia
O
..
..
..
..
..
..
HO
..
..
OH
..
H
O
C
R
O
..
..
..
C
..
..
O
R
O
..
..
..
..
C
addition
slow
elimination
deprotonation
–
..
O
..
..
FIGURE 18.39 The base-induced hydrolysis of amides gives carboxylate anions.
Acidification of the carboxylate at the end of the reaction would give the
carboxylic acid.
Loss of the amide ion (
NH
2
) gives the carboxylic acid, and deprotonation of the acid
gives the carboxylate anion. Loss of amide ion is surely difficult, because
NH
2
is a much stronger base than hydroxide. We know the relative base strengths by
comparing the pK
a
’s of ammonia and water. So the reverse reaction that regen-
erates the starting material is much faster. However, when an amide ion is lost,
a carboxylic acid is formed, and is quickly converted into the carboxylate. So, loss
of the amide ion, though slow, is essentially irreversible. A final acidification (not
shown in Fig. 18.39) regenerates the carboxylic acid in isolable form. Overall, it’s
addition–elimination once again.
PROBLEM 18.15 In strong base, the hydrolysis of an amide is second order in
hydroxide. In other words, two molecules of HO
are required to hydrolyze one
amide molecule. Write a modified mechanism to account for the need for two
molecules of hydroxide.
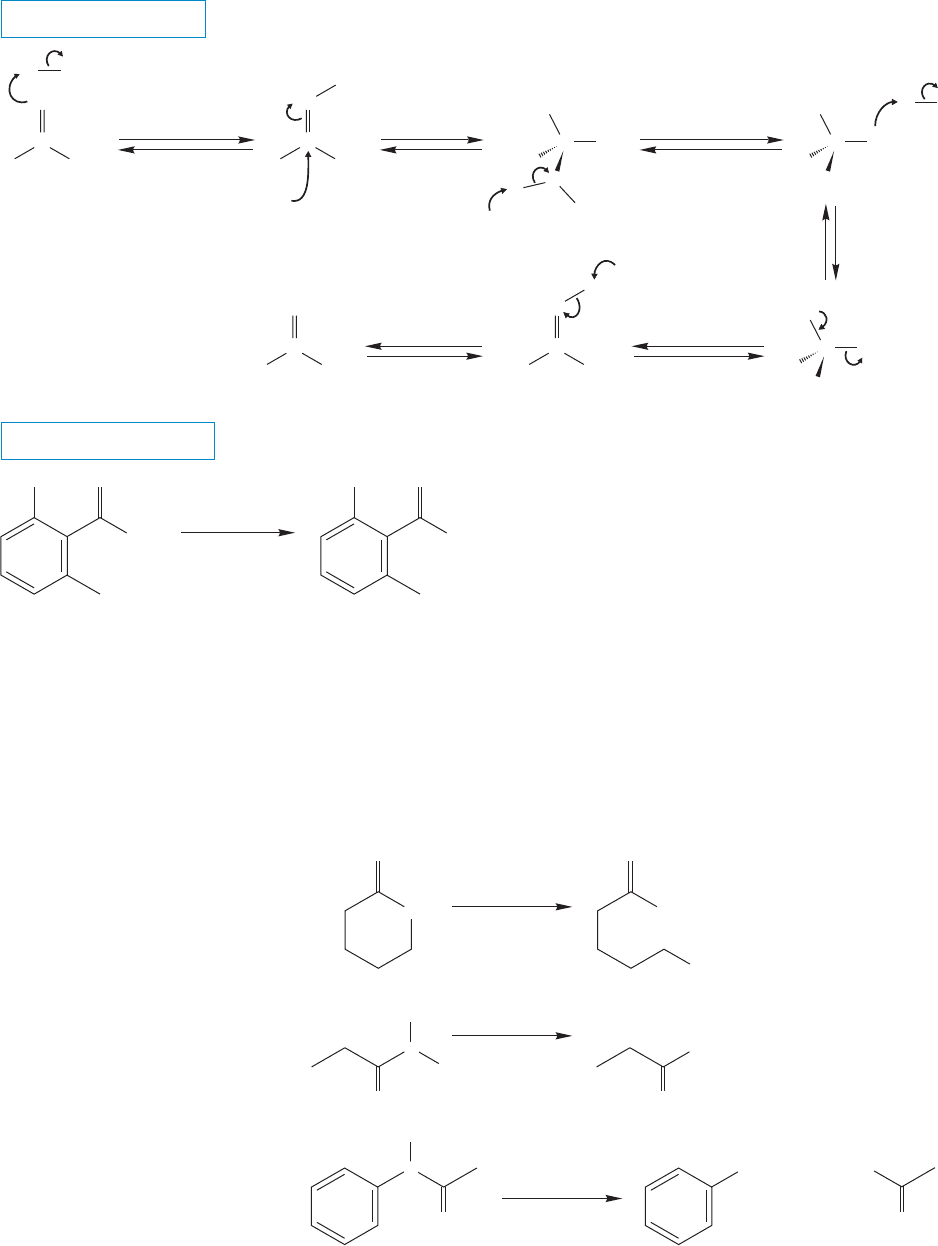
902 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
In acid, the carbonyl oxygen is protonated creating a Lewis acid strong enough
to react with the relatively weak nucleophile water (Fig. 18.40).The leaving group
OH
2
..
+
+
+
..
..
H
2
O
..
..
H
2
O
C
..
R
R
H
H
H
OH
2
..
+
H
NH
2
..
NH
2
O
..
..
..
O
..
HO
..
C
H
..
R
H
NH
2
O
..
C
+
..
..
ROH
O
..
..
C
protonation
addition
deprotonation
deprotonation elimination
protonation
C
R
..
NH
2
..
HO
..
..
HO
..
OH
2
..
+
C +
R
NH
3
NH
3
+
..
..
ROH
..
..
..
..
O
..
..
O
..
..
O
..
..
C
NH
4
..
HO
..
..
..
HO
..
THE GENERAL CASE
A SPECIFIC EXAMPLE
145 °C
(70%)
H
3
PO
4
/H
2
O
OHNH
2
FIGURE 18.40 The acid-induced hydrolysis of amides gives the carboxylic acid directly.
in the acid-induced reaction is the amine (Fig. 18.41), which makes this reaction
much easier than the base-induced hydrolysis. NH
3
is a better leaving group
than
NH
2
.
H
2
SO
4
/H
2
O
O
NH
..
..
O
OH
NH
3
..
..
..
..
+
+
+
H
2
SO
4
/H
2
O
H
2
SO
4
/H
2
O
N
..
..
..
..
..
(CH
3
)
2
NH
2
CH
3
CH
3
+
+
O
..
..
O
N
H
..
..
O
..
..
HO
OH
..
..
..
..
O
NH
3
FIGURE 18.41 Examples of amide
hydrolysis.
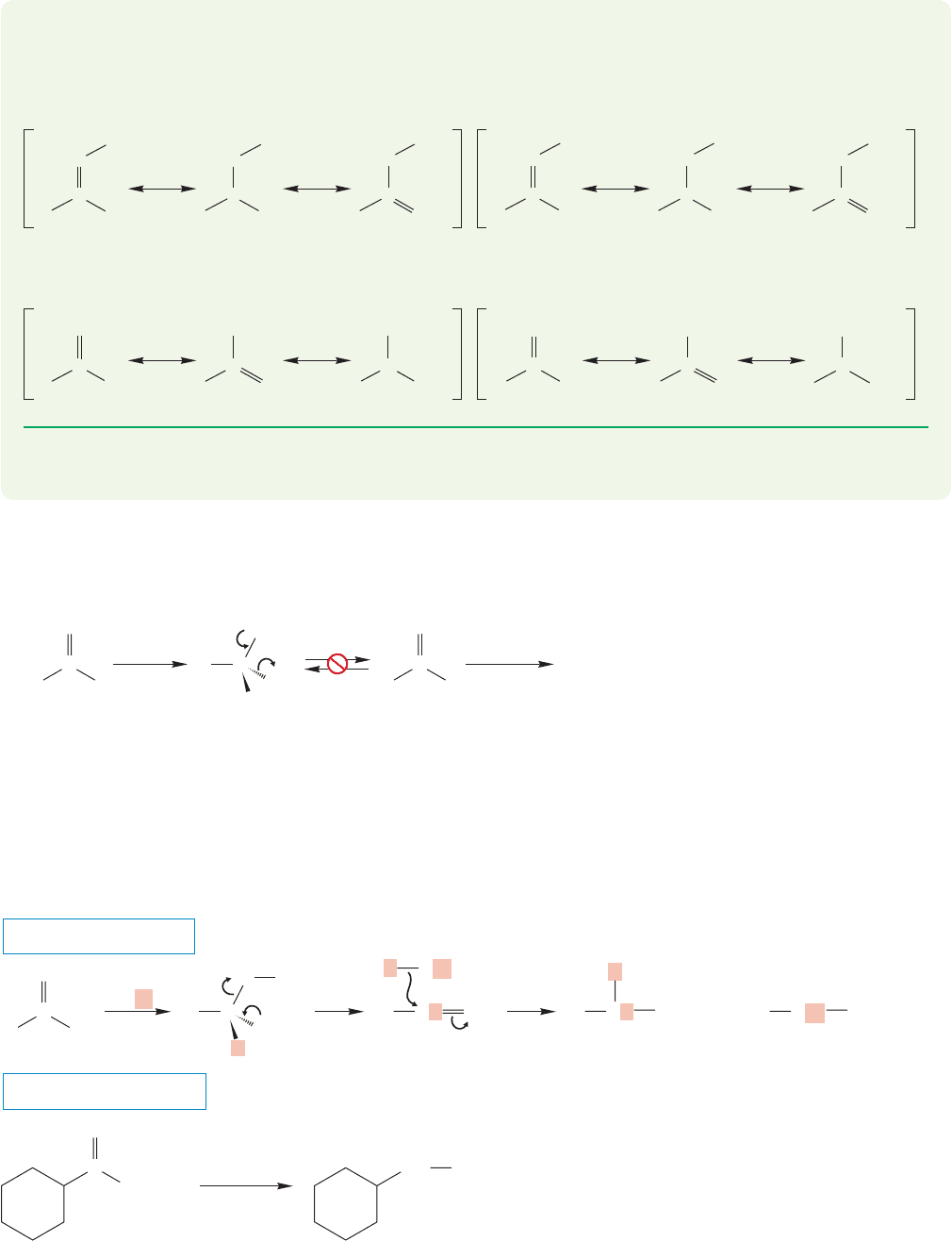
18.9 Reactions of Amides 903
WORKED PROBLEM 18.16 Write resonance forms for the structures in Figure 18.40
(The General Case) where appropriate.
ANSWER This problem is very similar to Problem 18.8. In this case, it is the proto-
nated amide and protonated carboxylic acid that are most stabilized by resonance.
O
H
..
C
NH
2
R
..
+
O
H
..
C
NH
2
R
..
+
..
O
H
..
C
NH
2
R
+
..
O
H
..
C
R
+
O
H
..
C
R
+
..
O
H
..
C
R
..
..
..
OH
..
..
OH
+
..
OH
The starting amide and acid are also resonance stabilized, but resonance stabiliza-
tion is more important for the charged intermediates in the reaction.
..
O
..
C
R
..
O
..
NH
2
NH
2
R
+
–
–
..
C
R
+
..
..
O
..
..
..
NH
2
..
O
..
C
R
O
..
C
R
+
–
–
..
C
R
+
..
..
O
..
..
..
OH
..
..
OH
..
OH
..
C
PROBLEM 18.17 Predict the organic products obtained from the acid-induced
hydrolysis of N-phenylpentanamide.
Metal hydrides reduce amides to amines (Fig. 18.42). Clearly, something new is
happening here, because analogy to the reactions of acids, esters, and acid chlorides
would lead us to expect alcohols as products.
C
..
..
..
R
R
NH
2
..
NH
2
O
..
..
H
..
..
H
C
R
O
..
..
C RCH
2
OH
LiAlH
4
1. LiAlH
4
2. H
2
O
–
..
O
..
..
FIGURE 18.42 By analogy to what we
know of other acyl compounds, we
might well expect the treatment of an
amide with a metal hydride to result
in the formation of an alcohol. In
fact, this reaction does not take place.
An amine is formed instead.
THE GENERAL CASE
A SPECIFIC EXAMPLE
–
+
C
N(CH
3
)
2
C
..
..
R
R
NH
2
..
..
..
NH
2
O
..
..
..
O
..
..
O AlH
2
H
..
C
H
H
Li
+
AlH
3
CH
N(CH
3
)
2
R
Iminium ion
NH
2
CHRNH
2
=
..
CH
2
Amine
RNH
2
LiAlH
4
LiAlH
4
ether
35 ⬚C, 1 h
CH
2
(88%)
FIGURE 18.43 Amines are the actual
products of the reduction of amides
with metal hydrides.
The mechanism of this reaction is complex and may vary with the number of
alkyl groups on the amide nitrogen. However, the first step is certainly the same as
in the other reductions by metal hydrides; the hydride adds to the carbonyl to give
an alkoxide that is coordinated to a metal (Fig. 18.43). Now, the metal oxide is lost
with formation of an iminium ion. There are several possible ways to describe the
formation of the iminium ion,but once it is produced, reduction by the metal hydride
to give the amine is sure to be rapid.
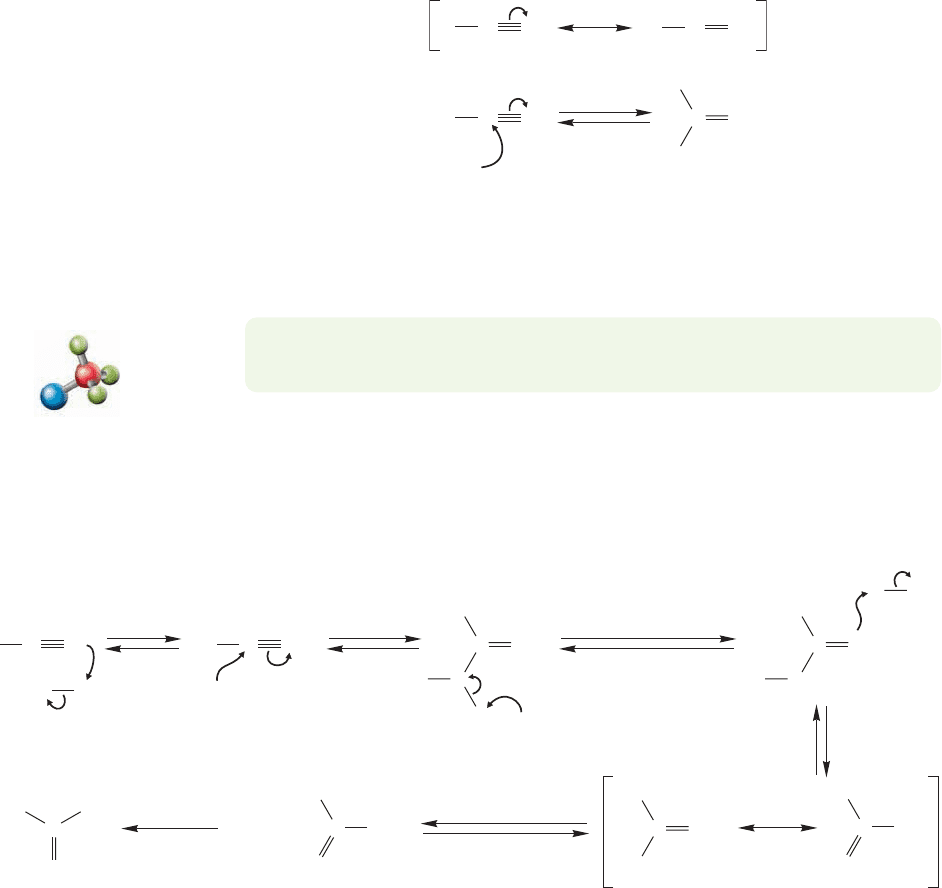
904 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
R
R
Nu
CN
RC
C
Imine anion
N
–
..
..
..
–
+
..
RC N
..
..
N
Nu
..
–
addition
FIGURE 18.44 Nitriles are
electrophiles, and nucleophiles add
to them to give imine anions.There
is a close analogy to carbonyl
chemistry here.
PROBLEM 18.18 Explain why an sp-hybridized nitrogen should be a weaker base
than an sp
2
-hybridized nitrogen. What about sp
3
-hybridized nitrogens?
Both acid- and base-induced hydrolysis of a nitrile gives the amide,but rather severe
conditions are required for the reactions, and further hydrolysis of the intermediate
amides gives the carboxylic acids (p. 902). In acid, the first step is protonation of the
nitrile nitrogen to give a strong Lewis acid (A, Fig. 18.45), which is attacked by water.
A series of proton shifts then gives an amide that is hydrolyzed to the carboxylic acid.
+
H
3
O
protonation
addition
deprotonation
protonation
..
+
H
3
O
..
..
+
+
+
+
+
+
..
..
..
..
H
2
O
..
..
/H
2
O
..
..
H
2
O
deprotonation
hydrolysis
..
..
..
..
H
2
O
..
..
..
..
..
..
OH
2
..
..
OH
H
3
O
..
+
..
..
H
2
O
..
H
2
O
..
RC
H
N
R
R
OH
..
OH
2
H
H
CC
A
NH NH
..
..
R
OH
CNH
R
HO
CNH
2
+
R
HO
CNH
2
..
R
O
C
..
..
R
O
C
Carboxylic acid
(Fig. 18.40)
Amide
(not stable under
these conditions)
NH
2
FIGURE 18.45 The acid-induced hydrolysis of a nitrile to a carboxylic acid.The nitrile is the Lewis base (nucleophile) in
the first step.
Nitrile hydrolysis
18.10 Reactions of Nitriles
Of course a nitrile is not a carbonyl. But nitrile and carbonyl chemistries are similar,
and an examination of nitrile reactions naturally belongs in this section. Like carbonyl
compounds, nitriles are both electrophiles and nucleophiles. As in carbonyl com-
pounds, a polar resonance form contributes to the structure of nitriles (Fig. 18.44).
Consequently, the triple bond is polarized and the carbon acts as a Lewis acid. One
might anticipate that nucleophiles would add to nitriles,and that idea is correct.These
similarities mean that many of the reactions of carbonyl groups are also possible for
nitriles, and the mechanisms are generally closely related. Students sometimes have
trouble with the nitrile reactions,however,so it is important not to dismiss them with
only a cursory look. For some reason, the analogy between the carbon–oxygen dou-
ble bond and the carbon–nitrogen triple bond is not always easy to keep in mind.
The nitrogen atom of the nitrile bears a pair of nonbonding electrons and is
therefore the center of nucleophilicity and Brønsted basicity. The nitrogen is
sp-hybridized, however, and is a relatively weak base.
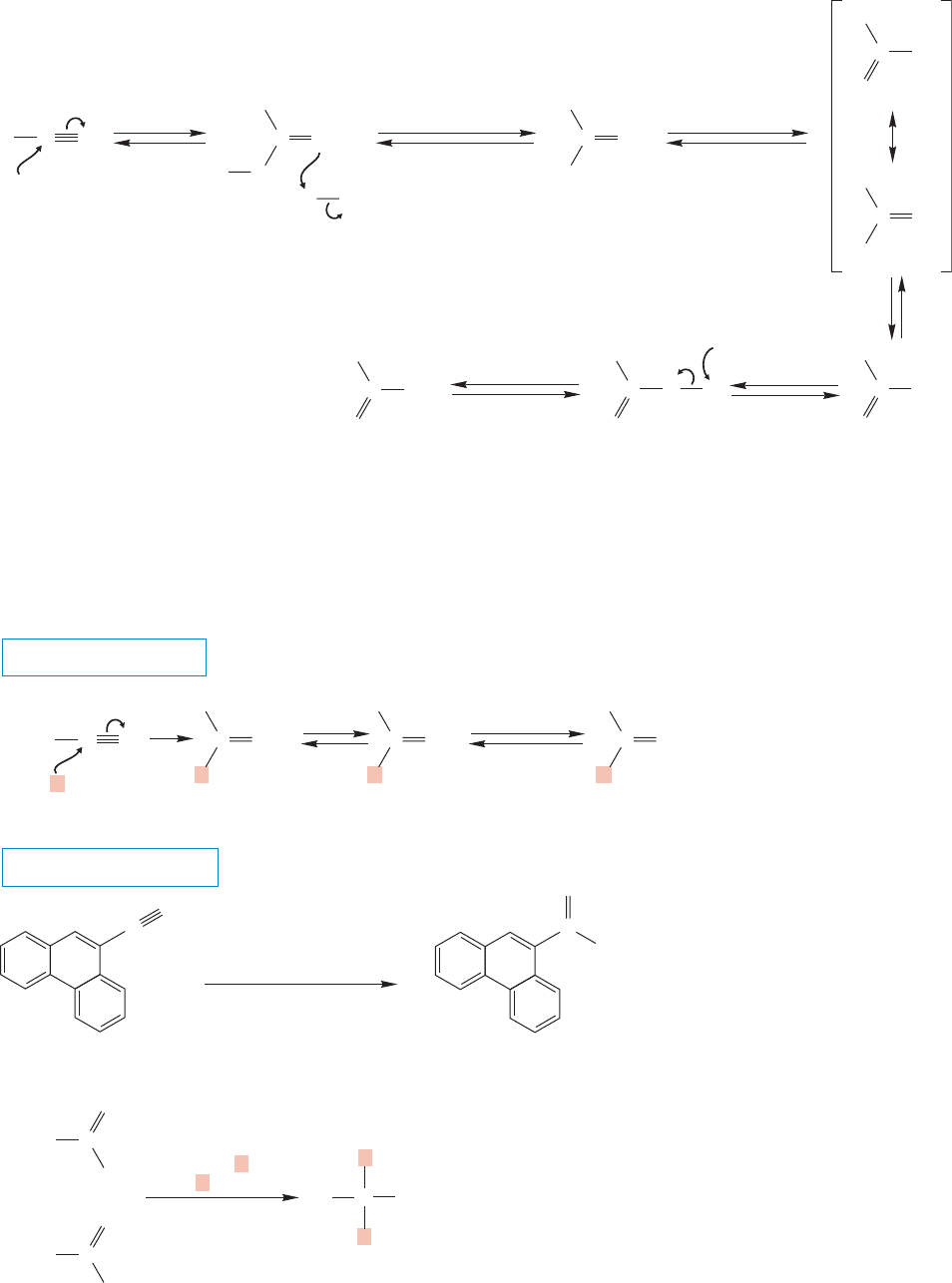
18.10 Reactions of Nitriles 905
In base, the nucleophilic hydroxide ion adds to the carbon–nitrogen triple bond
(Fig. 18.46). Protonation on nitrogen and deprotonation from oxygen leads to the
amidate anion, which is protonated to give an intermediate amide. Basic hydrolysis
of the intermediate amide leads to the carboxylate salt.
+
addition
protonation
deprotonation
deprotonation
–
–
..
..
..
..
..
..
..
..
H
2
O
hydrolysis
..
..
..
..
H
2
O
..
..
..
..
..
..
..
..
..
..
..
H
2
O
..
..
HO
–
–
–
..
..
..
OH
–
..
..
..
OH
–
..
..
RCN
R
OH
..
..
OHH
CN
..
R
HO
CNH
R
O
CNH
R
O
CNH
..
R
O
C
Carboxylate
(Fig. 18.39)
Amide
NH
2
..
..
..
..
R
O
CO
..
..
..
..
R
O
COH
FIGURE 18.46 The base-induced hydrolysis of a nitrile to give a carboxylate anion.The nitrile is the Lewis acid (electrophile)
in the first step.
Stronger bases also add to nitriles. Organometallic reagents give ketones,
although the product before the hydrolysis step is the imine (Fig. 18.47a).
Addition to the nitrile first takes place in normal fashion to give the imine salt.
THE GENERAL CASE
A SPECIFIC EXAMPLE
(b) Recall reactions of esters and acid chlorides
/H
3
O
..
+
–
+
..
..
..
..
H
2
O
..
..
1. 2 equiv. RMgX
(or RLi)
2. H
2
O
..
..
..
O
..
..
H
2
O
..
RC
RLi
N
R
R
CN
..
RC
OR
or
..
..
..
..
O
..
RC
Cl
..
R
R
C
Imine salt Imine Ketone
NH
..
NH
3
..
..
R
R
CROH
..
..
R
R
C
+O
Li
1. CH
3
MgI
benzene, 80 ⬚C, 3 h
2. H
2
O/HCl, 0 ⬚C
..
N
..
..
C
C
(55%)
O
CH
3
(a)
hydrolysis
FIGURE 18.47 (a) Reaction of a
nitrile with an organometallic reagent
gives an imine salt. Acidification first
generates the imine and then the
ketone.The organometallic reagent
adds once. (b) Reaction of an ester or
acid chloride with organometallic
reagents gives an alcohol.The
organometallic reagent adds twice.
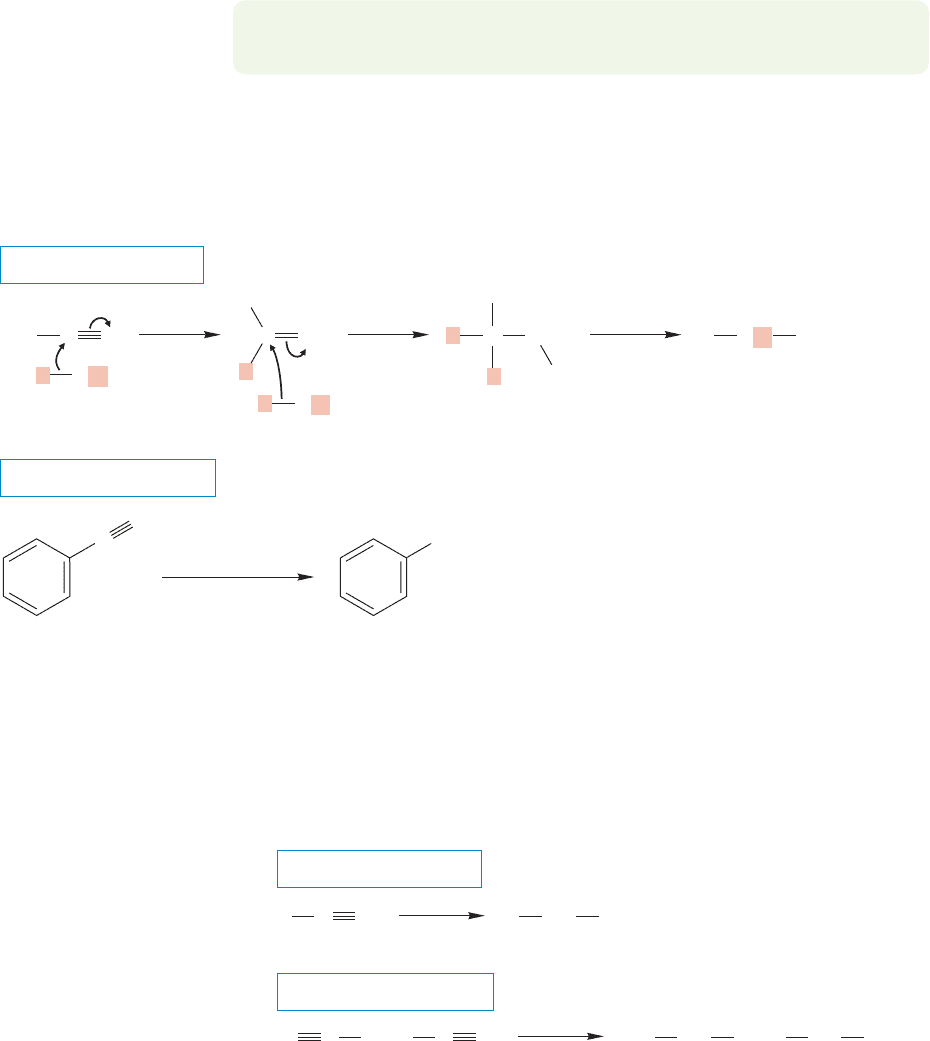
906 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
PROBLEM 18.19 Write a mechanism for the hydrolysis of imines under neutral
conditions to give a ketone (Fig. 18.47).
When the reaction is worked up with water, protonation gives the imine, which in
acid undergoes further hydrolysis to the ketone. Note the difference between this
reaction and those of alkyllithium or Grignard reagents with acid chlorides or esters,
which go all the way to alcohols. With nitriles the reaction can be stopped at the
carbonyl stage (Fig. 18.47a). With acid chlorides and esters the intermediate car-
bonyl compounds are born in the presence of the organometallic reagent and must
react further. In the reaction with nitriles, there is no leaving group present and the
imine salt is stable until hydrolysis.
Metal hydrides reduce nitriles to give primary amines (Fig. 18.48). Two
equivalents of hydride are delivered, and subsequent addition of water yields the
amine.
THE GENERAL CASE
A SPECIFIC EXAMPLE
–
+
..
..
..
..
H
2
O
1. LiAlH
4
THF, 0 ⬚C, 1 h
2. H
2
O
..
RC
H
AlH
3
N C
(72%)
R
..
N
R
AlH
2
H
H
H
CH
2
NH
2
Al(OH)
3
R
C
C
CH
2
NH
2
Li
+
H
AlH
2
Li
–
+
..
..
N
Li
–
+
..
..
N
Li
addition addition
hydrolysis
+
FIGURE 18.48 The metal hydride reduction of nitriles followed by hydrolysis yields primary amines.
THE GENERAL CASE
A SPECIFIC EXAMPLE
..
RC
H
2
catalyst
N
..
.. ..
RCH
2
Primary amine
NH
2
..
CC(CH
2
)
8
Raney Ni/H
2
125 ⬚C
N
..
N CH
2
CH
2
(CH
2
)
8
(80%)
H
2
N NH
2
FIGURE 18.49 Catalytic
hydrogenation of nitriles gives
primary amines.
Catalytic hydrogenation of nitriles also gives primary amines (Fig.18.49). Notice
that only primary amines can be formed this way. Alkenes are more reactive toward
typical hydrogenation conditions than are nitriles.
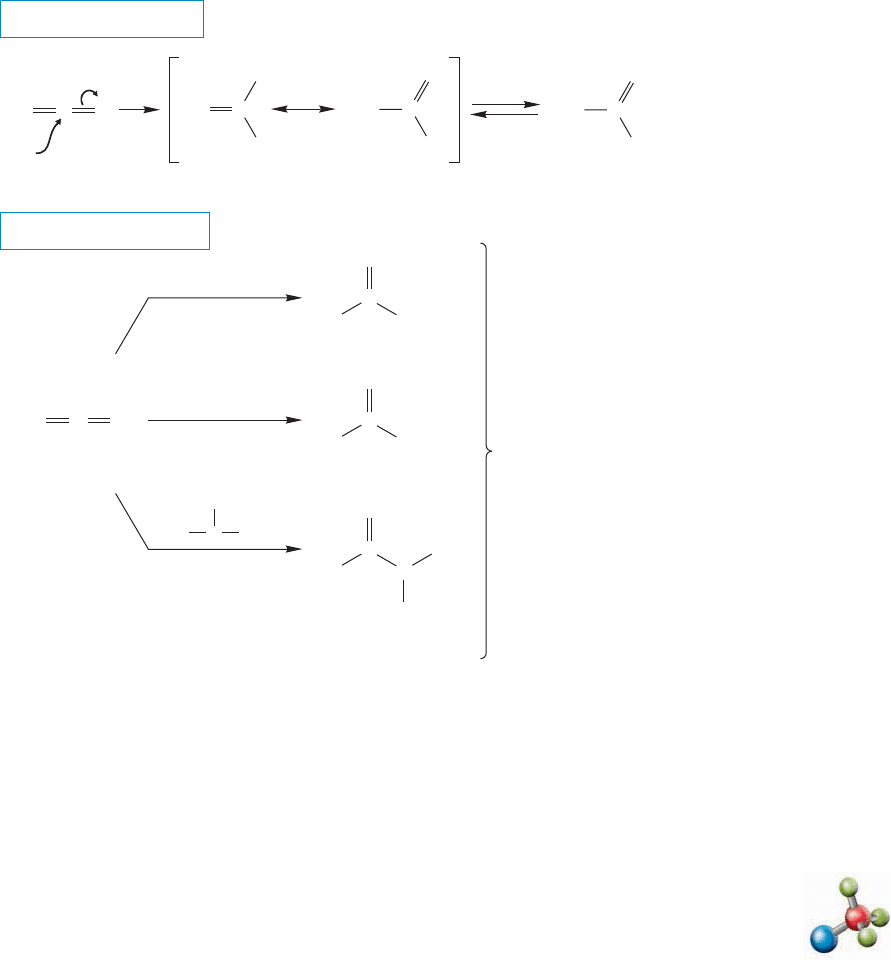
18.12 Special Topic: Other Synthetic Routes to Acid Derivatives 907
THE GENERAL CASE
SPECIFIC EXAMPLES
OC
..
O
..
..
H
3
O
..
+
Nu
Nu
..
..
..
–
–
H
2
C
OC
..
..
..
..
H
2
C
CH
3
CH
3
N
..
Ph
C
H
2
C
O
..
..
Nu
NuH
..
–
CH
2
C
O
..
..
C
(93%)
H
3
C
O
..
Nu
..
C
Acyl
compounds
Acyl compounds
An enolate
H
3
C
CH
3
CH
2
SH
..
..
SCH
2
CH
3
(CH
3
)
3
COH
–80 ⬚C, 3 days
..
..
O
..
..
C
(87%)
H
3
C
..
..
OC(CH
3
)
3
O
..
..
C
(75%)
H
3
C
..
N
Ph
ethyl alcohol
water
H
FIGURE 18.50 Ketenes react with
nucleophiles to give acyl compounds.
18.11 Reactions of Ketenes
Ketenes have a very electrophilic sp-hybridized carbon and are therefore reactive
even at low temperature. Most ketenes are so reactive that they must be carefully
protected from atmospheric moisture. Nucleophiles add to the carbonyl group to
give acyl derivatives. This addition is another reaction that gives students trouble,
but shouldn’t.The ketene reacts with nucleophiles like any other carbonyl compound
to give an addition product (Fig. 18.50). In this case, the addition product is an eno-
late (p. 373), and protonation gives the stable carbonyl compound.Figure 18.50 gives
some examples.
18.12 Special Topic: Other Synthetic Routes
to Acid Derivatives
As mentioned in the introduction to this chapter, reactions and syntheses are even
more intertwined than usual with these acid derivatives. A reaction of one is a syn-
thesis of another. The various interconversions of acyl compounds discussed in this
chapter (and in Chapter 17) are summarized in Section 18.15.Here we first discuss
two rearrangement reactions that are useful as syntheses of esters and amides, respec-
tively, then move on to a brief discussion of routes to nitriles and ketenes.
18.12a The Baeyer–Villiger Reaction Named by Kurt Mislow (p. 152) for
Adolf von Baeyer (p. 187) and his student Victor Villiger (1868–1934), the
Baeyer–Villiger reaction is an oxidation of aldehydes and ketones, usually with a
Baeyer–Villiger reaction
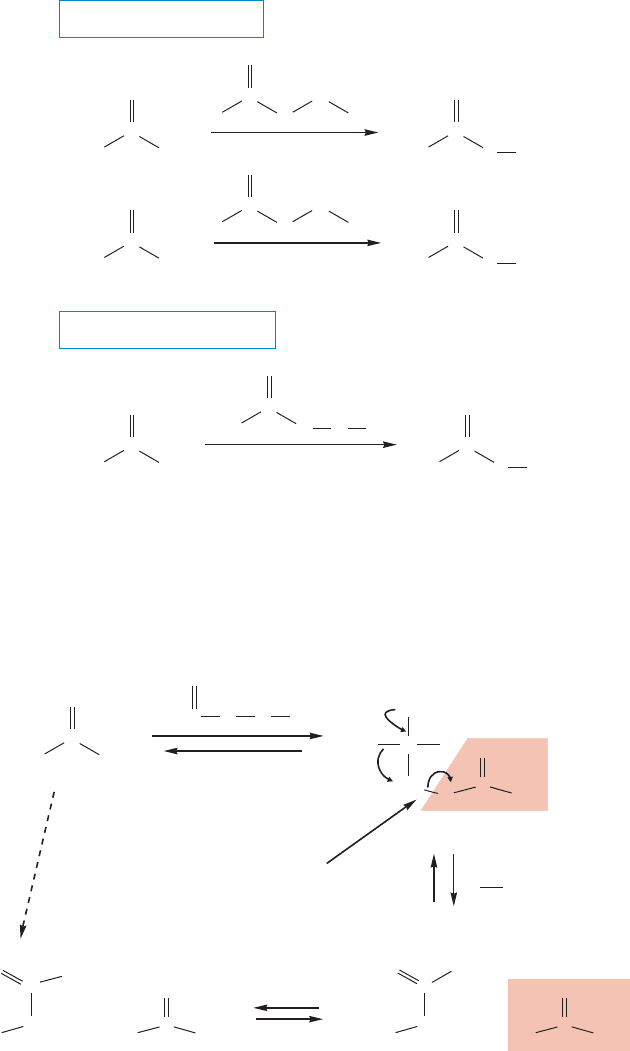
908 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
peracid (p. 424), that results in the insertion of an oxygen atom next to the carbonyl
carbon (Fig. 18.51). Seen in its bare form, the reaction appears quite remarkable—how
can that “extra” oxygen atom insert itself into a carbon–carbon bond? Fleshed out in a
..
..
..
..
THE GENERAL CASE
A SPECIFIC EXAMPLE
R
25 ⬚C, 10 days
(86%)
O
C
R
R
O
C
O
R
H
O
C
H
R
O
C
O
R
Ph
O
C
Ph
Ph
O
C
O
Ph
O
O
C
R
O H
O
O
C
R
O H
O
C
Ph
OOH
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
FIGURE 18.51 Baeyer–Villiger
oxidation of aldehydes and ketones.
C
OH
RR
C
CF
3
3
C
HO
++
+
R
O
R
CF C
proton
transfer
R moves as the
O
O bond breaks
Weak
bond
Good leaving
group
overall transformation
= insertion of one
oxygen atom
C
O
R
O
R
O
O
C
CF
3
O
HO
C
CF
3
O
O
–
..
O
C
R
R
..
..
..
..
O
..
..
..
..
..
..
..
OOH
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
O
..
..
FIGURE 18.52 The mechanism
of the Baeyer–Villiger reaction.
real mechanism, however, the reaction becomes intelligible.The first stage is a straight-
forward addition of the peracid to the carbonyl group (Fig. 18.52). Now look at that the
weak oxygen–oxygen bond, and at the good leaving group formed when it breaks.
Migration of an R group as the oxygen–oxygen bond breaks gives the product directly.
Of course when there are two different R groups in the ketone, two products
can result, because either R group can migrate. However, there is a well-established
order of migration for different groups: H tertiary alkyl secondary alkyl pri-
mary alkyl methyl, and there is quite substantial selectivity in product formation.
That is why aldehydes give the acid, as shown in Figure 18.51. The hydrogen
migrates in preference to the alkyl or aryl group.
