Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

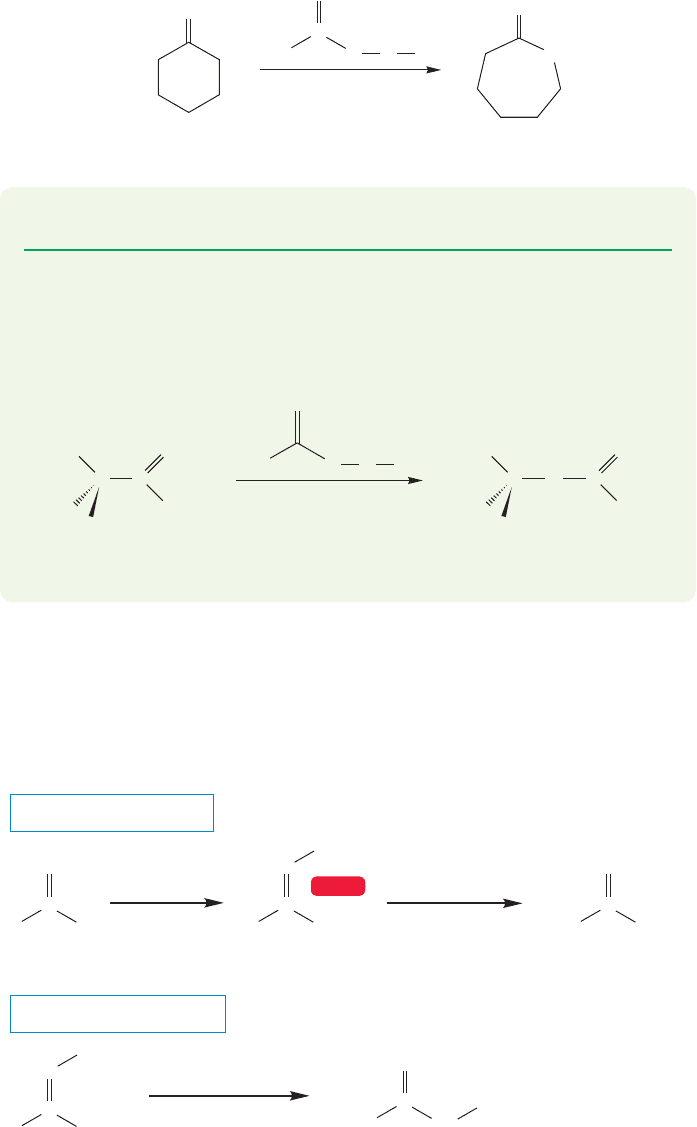
18.12 Special Topic: Other Synthetic Routes to Acid Derivatives 909
When the ketone is cyclic, the Baeyer–Villiger reaction becomes a synthesis of
lactones (p. 849). Watch out for this reaction.The cyclic ketone adds apparent com-
plexity, and this reaction is a favorite of problem writers (Fig. 18.53). Nevertheless,
the mechanism is the same for the cyclic ketone as you will see in Problem 18.20.
25 °C, CHCl
3
(71%)
O
O
O
O
C
Ph
OOH
..
..
..
..
..
..
..
..
..
..
..
..
FIGURE 18.53 When a cyclic ketone
is used, the Baeyer–Villiger reaction
becomes a synthesis of lactones.
PROBLEM 18.20 Write a mechanism for the reaction in Figure 18.53.
PROBLEM 18.21 In the paper that named the Baeyer–Villiger reaction, Mislow
and his student Joseph Brenner showed that in the reaction below, the product
was formed with retention of optical activity. What does that observation tell you
about the details of the mechanism?
25 °C, 65 h, CHCl
3
Ph
H
(S )(S )
C
O
H
3
C
CH
3
C
Ph
H
C
O
H
3
C
CH
3
CO
*
*
O
Ph
OOH
..
..
..
..
..
..
..
..
..
..
..
..
18.12b The Beckmann Rearrangement Here is a related reaction called the
Beckmann rearrangement, named for Ernst Otto Beckmann (1853–1923), in
which the end product is not an ester, but an amide.The first step is formation of an
oxime (p. 793), the product of the addition of hydroxylamine to a ketone (Fig.18.54).
Treatment of the oxime with any of a variety of strong acids leads to the amide.
O
..
..
..
..
THE GENERAL CASE
A SPECIFIC EXAMPLE
An oxime An amide
O
C
R
R
O
C
NH—R
R
N
OH
C
R
R
H
2
SO
4
H
2
O
NH
2
OH
H
3
O
+
(100%)
polyphosphoric
acid
xylene, 100 °C
2 h
N
C
Ph
Ph
Ph
C
NH
Ph
WEB 3D
..
..
..
..
..
..
OH
..
..
..
..
FIGURE 18.54 The Beckmann
rearrangement converts a ketone
into an amide.
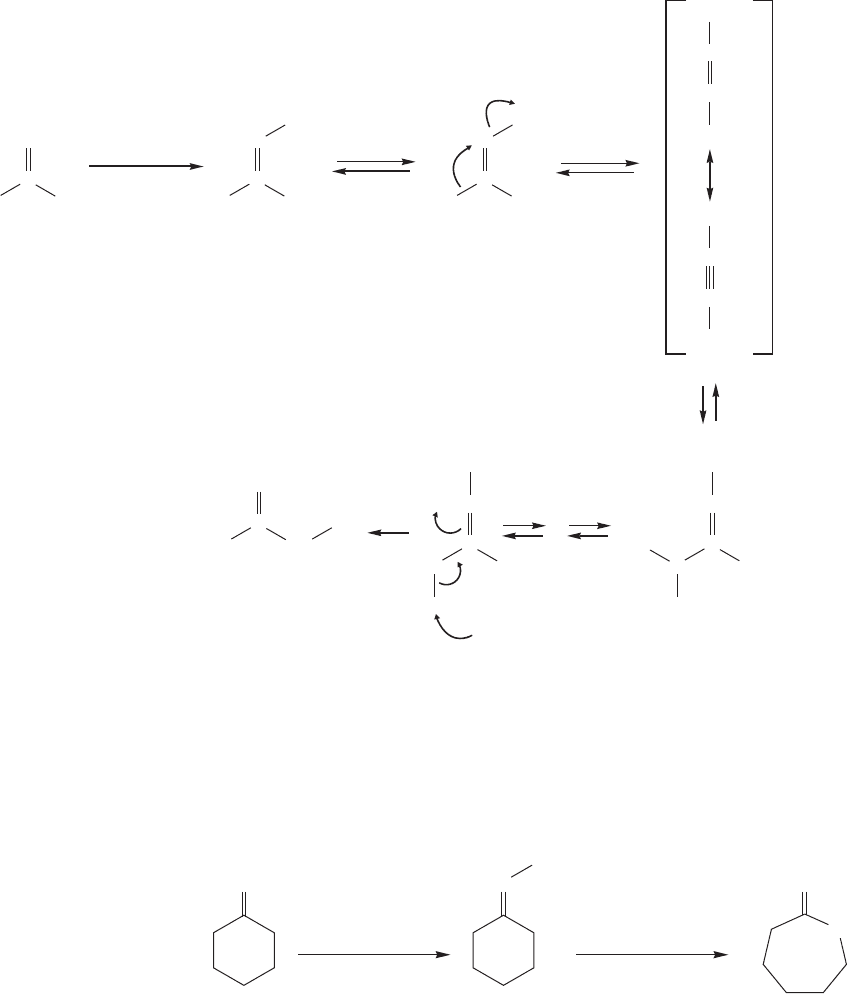
910 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
The mechanism of the Beckmann rearrangement (Fig. 18.55) involves a migra-
tion much like the one in the Baeyer–Villiger reaction, but the reaction is not trig-
gered by the breaking of a weak oxygen–oxygen bond. Instead, it is initiated by the
displacement of a good leaving group, water. That good leaving group is formed as
the OH of the oxime is protonated by acid. Intramolecular rearrangement leads to
a nicely resonance-stabilized intermediate that is then captured by water. Proton
shifts lead to the final product, the amide.
..
O
..
..
O
..
.. .. ..
..
..
..
.. ..
..
Ph
C
N
+
+
+
+
+
H
H
H
3
2
O
H
2
O
OH
2
proton
transfers
+ H
2
O
N
OH
C
Ph
N
Ph
Ph
Ph
Ph
Ph
C
Ph
C
Ph
Ph
C
NH
Ph
N
C
Ph
O
+
H
Ph
NH
C
Ph
O
N
OH
C
Ph
Ph
H
3
O
+
/NH
2
OH
..
..
..
..
..
..
..
..
..
..
..
..
..
FIGURE 18.55 The mechanism of the Beckmann rearrangement.
As shown in Figure 18.56, in its cyclic version, the Beckmann rearrangement
leads to a lactam (p. 851).
..
NH
polyphosphoric
acid
100 ⬚C, 4 h
(90%)
O
NO
OH
NH
2
OH
H
3
O
+
..
..
..
..
..
..
..
..
..
..
..
FIGURE 18.56 When cyclic ketones are used, the Beckmann rearrangement
becomes a synthesis of lactams.
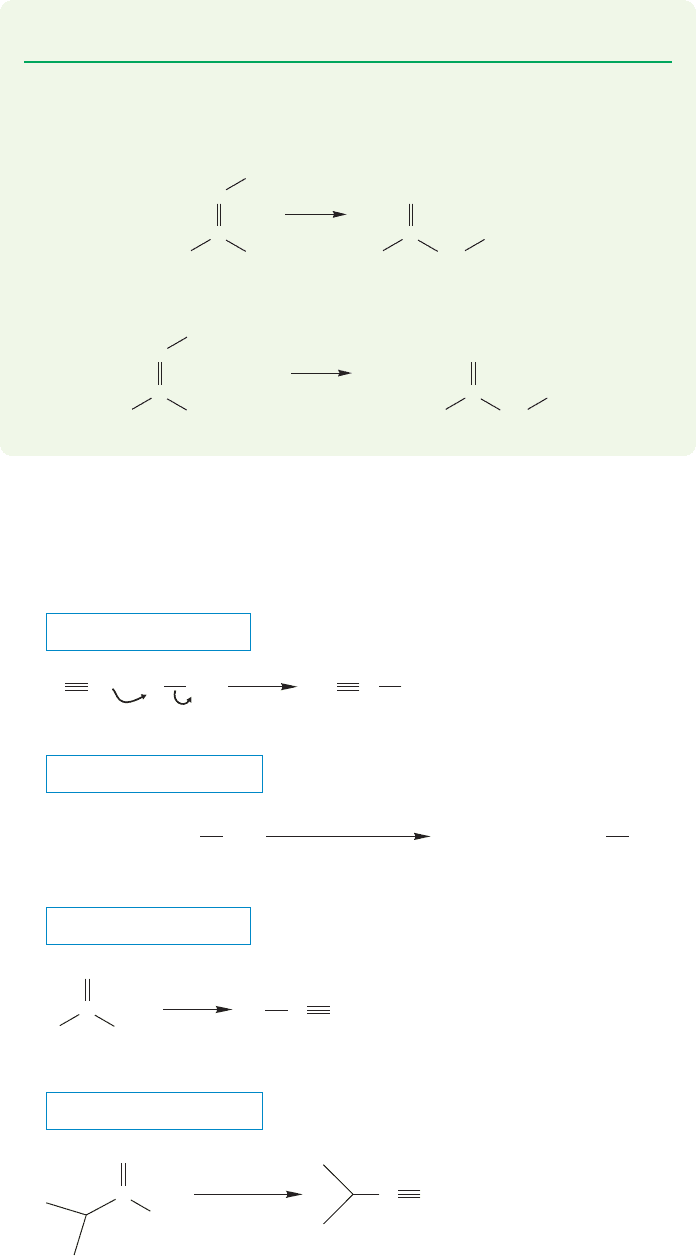
18.12 Special Topic: Other Synthetic Routes to Acid Derivatives 911
PROBLEM 18.22 Provide a mechanism for the reaction shown in Figure 18.56.
PROBLEM 18.23 Oximes can be obtained as the E or the Z isomers, and the two
stereoisomers react differently. Explain the results shown here:
18.12c Nitrile Formation Nitriles are most often made by displacement reac-
tions of alkyl halides by cyanide ion or by dehydration of amides, generally with
P
2
O
5
(Fig. 18.57).
..
N
OH
C
CH
3
CH
3
CH
2
CH
2
O
C
NHH
3
C
CH
2
CH
2
CH
3
H
3
O
+
C
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
O
C
NHH
3
C
CH
3
H
3
O
+
..
..
..
N
OH
..
..
..
..
..
..
..
..
..
..
A SPECIFIC EXAMPLE
..
C
(~75%)
N
P
2
O
5
210 ⬚C, 9 h
O
..
..
C
NH
2
..
THE GENERAL CASE
A SPECIFIC EXAMPLE
+
Cl CN
(93%)
..
..
..
NaCN
dimethyl sulfoxide
20 min, <160 ⬚C
..
..
..
CNR
CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
O
..
..
C
NH
2
P
2
O
5
⌬
R
..
..
..
H
2
O
THE GENERAL CASE
+
–
–
..
..
CN XX
S
N
2
R
..
..
CN R
FIGURE 18.57 Nitriles are usually made through S
N
2 reactions using cyanide as the
nucleophile. Nitriles can also be formed by dehydration of amides.
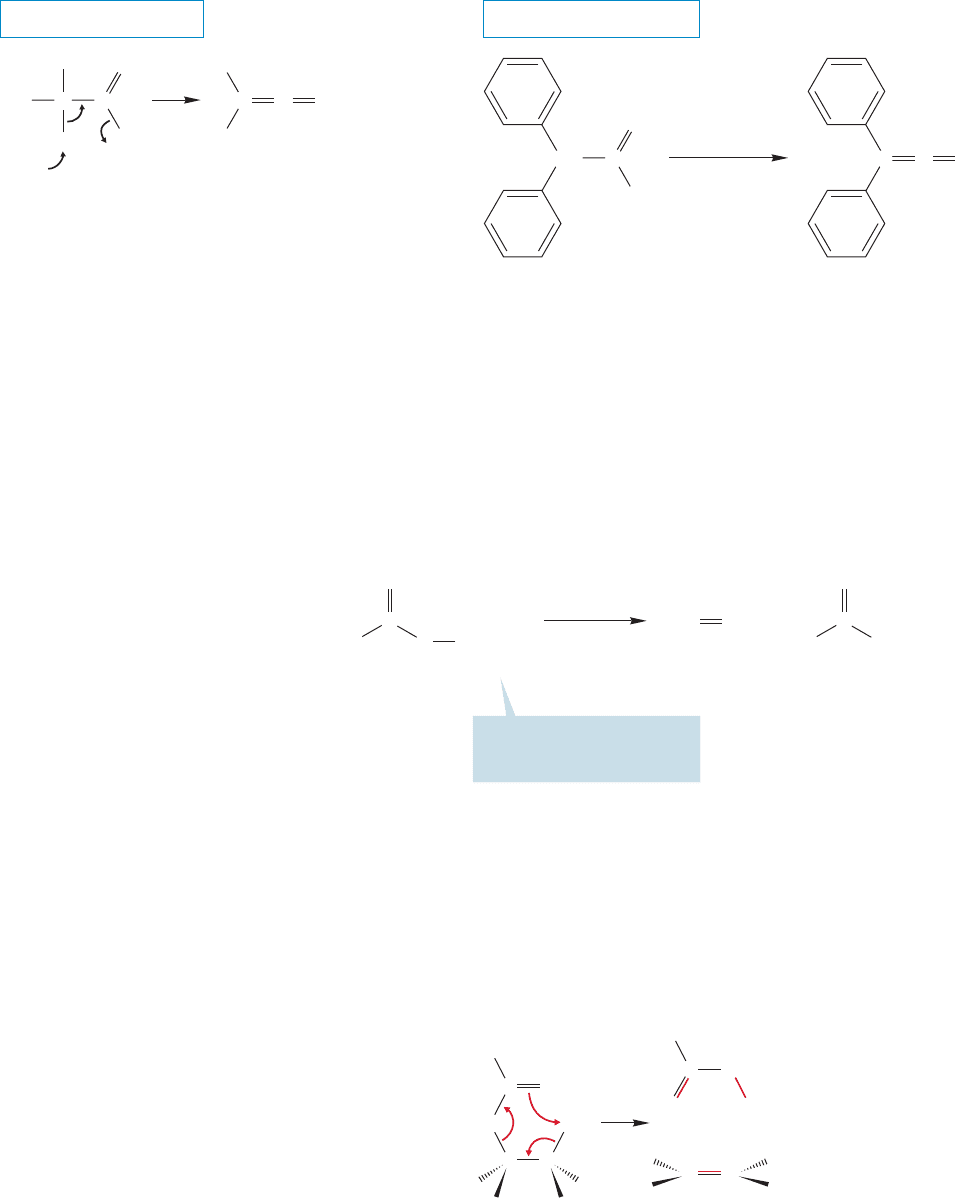
912 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
18.12d Ketene Formation Ketenes are synthesized through elimination
reactions of acyl chlorides using tertiary amines as bases (Fig. 18.58).
THE GENERAL CASE
A SPECIFIC EXAMPLE
+ Cl
..
..
..
..
Cl
..
..
..
..
..
..
..
..
..
–
+
C
H
R
R
R
R
R
3
N
R
3
NHCCC
A ketene
O
..
..
C O
O
Cl
..
..
..
..
..
CH C
O
Et
3
N , 0 ⬚C
ether
C
FIGURE 18.58 One common synthesis of ketenes involves an elimination of acyl chlorides.
18.13 Special Topic: Thermal Elimination Reactions
of Esters
Esters that contain a hydrogen in the β position of the OR group give alkenes
and carboxylic acids when heated to high temperatures (400 °C) (Fig. 18.59).
CH
2
+
For this reaction to succeed,
there must be a hydrogen
here, in the “” position
O
..
..
C
OCH
2
CH
3
H
2
C
␣
R
..
..
O
..
..
C
R
..
..
~400 ⬚C
gas phase
OH
FIGURE 18.59 The thermal
elimination reaction of esters
containing a hydrogen in the
β position.
In this reaction, the carbonyl group acts as a base, attacking the β hydrogen
intramolecularly. At the same time the ester acts as a leaving group, and a carboxylic
acid is lost in a single, concerted step (Fig. 18.60). In contrast to most E2 reac-
tions, this pyrolytic, intramolecular version proceeds through a syn elimination.
The reason is simple: Only the syn hydrogen is within reach of the carbonyl group.
HH
HH
C
C
C
O
R
H
H
H
..
..
..
..
O
C
OH
R
..
..
..
..
O
C
C
H
H
FIGURE 18.60 An arrow formalism
description of the thermal
elimination reaction of esters.
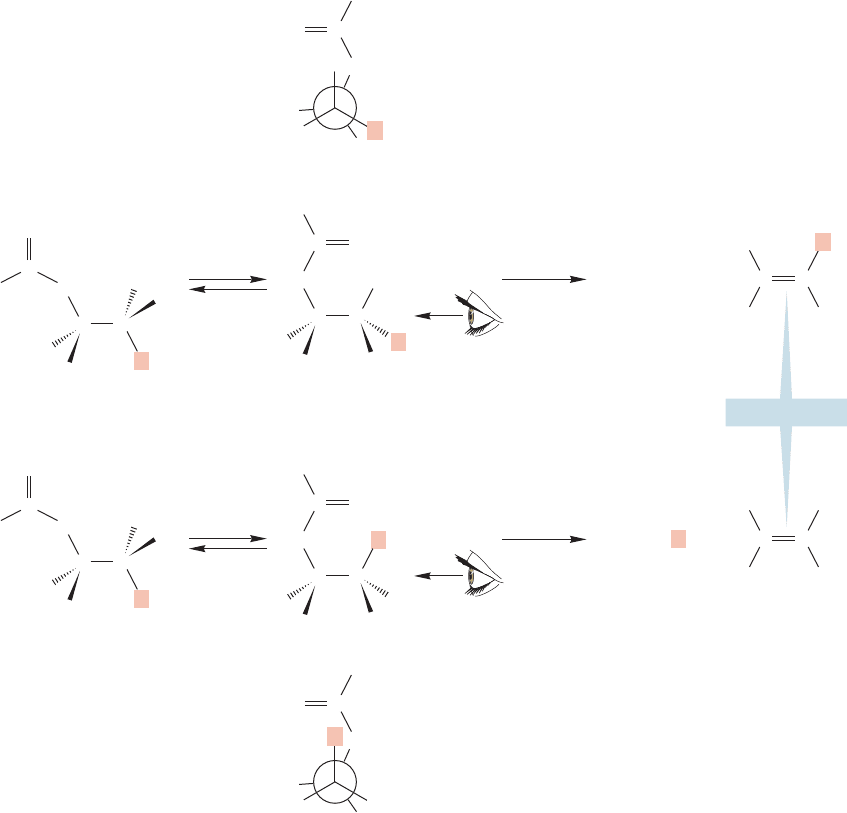
18.13 Special Topic: Thermal Elimination Reactions of Esters 913
There is no possibility of doing an anti elimination in this reaction. The elimi-
nation reactions of the deuterium-labeled compounds shown in Figure 18.61
provide an example of the stereospecificity of this elimination reaction. In
diastereomer A, it is only the cis hydrogen that is lost as trans-stilbene is formed.
By contrast, in isomer B it is the deuterium atom that must occupy the cis
position in the formation of trans-stilbene, and this time it is D, not H, that
is lost.
A
trans-Stilbene
rotation
..
..
rotation
Ph
Ph
Ph
H
D
D
C
C
C
OH
3
C
H
..
..
O
C
C
C
O
H
3
C
H
CC
H
Ph
Ph
..
..
..
..
O
C
CH
3
O
Ph
H
Newman projection
O
H
Ph
D
D
B
Syn elimination
500 ⬚C
Syn elimination
500 ⬚C
H and ester cis
D and ester cis
..
..
Ph
Ph
Ph
Ph
H
D
D
C
Diastereomeric starting materials
C
C
OH
3
C
H
..
..
O
H
C
C
C
O
H
3
C
H
CC
H
H
Ph
Ph
..
..
..
..
O
C
CH
3
O
Ph
H
Newman projection
O
H
Ph
D
Ph
H
CH
3
COOH
CH
3
COOD
+
+
FIGURE 18.61 In these intramolecular elimination reactions, only a cis hydrogen is
removed. Because the phenyl groups are required to stay as far from each other as possible
for steric reasons, syn elimination demands that H be lost from stereoisomer A and D lost
from stereoisomer B.
There are other versions of this new elimination process, some of which
proceed under much milder conditions than those necessary for ester pyrolysis.
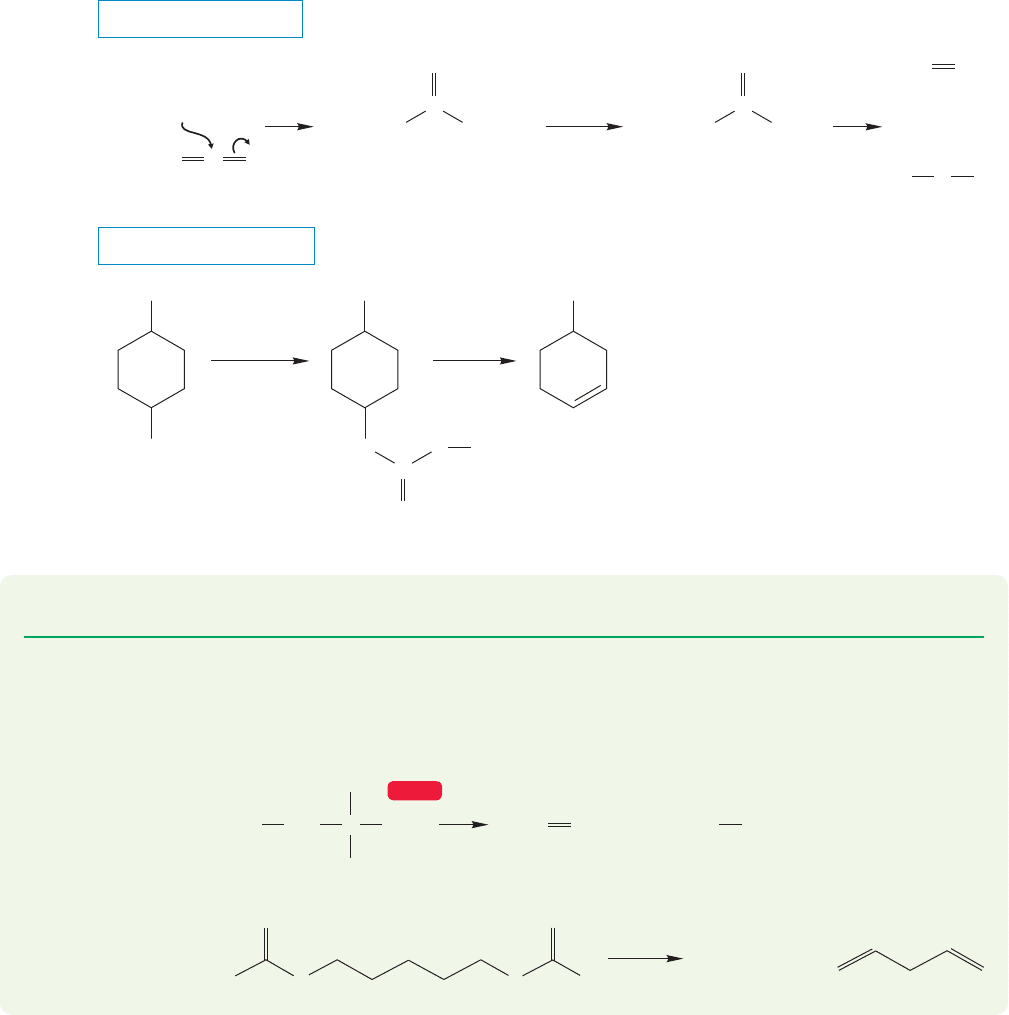
914 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
For example, the sulfur-containing xanthate esters undergo elimination especially
easily (Fig. 18.62). Xanthate esters can be prepared as shown in the figure by
addition of alkoxide to CS
2
(recall the addition of nucleophiles to carbon dioxide,
p. 840). These intramolecular thermal elimination reactions provide a new route
to alkenes, so update your file cards.
THE GENERAL CASE
A SPECIFIC EXAMPLE
C
+
COS
+
Na
+
Na
+
208 ⬚C
–
..
..
..
–
..
..
..
..
CH
3
CH
2
O
..
..
CH
3
CH
2
O
..
..
CH
3
I
S
N
2
..
..
..
..
SS
S
..
..
S
C
A xanthate ester
..
..
..
..
CH
3
CH
2
O
CH
2
S
..
..
SCH
3
C
(62%)
..
..
CH
3
S
..
..
S
..
..
S
C
CH
3
H
H
2
C
Carbon disulfide
OH
1. NaH
2. CS
2
3. CH
3
I
..
..
O
..
..
C(CH
3
)
3
C(CH
3
)
3
C(CH
3
)
3
FIGURE 18.62 The formation and thermal
fragmentation of a xanthate ester.
PROBLEM 18.24 Write arrow formalisms for the general reaction of Figure 18.62.
PROBLEM 18.25 Provide mechanisms for the following reactions. The first reac-
tion (the thermal elimination of an N-oxide) is called the Cope elimination, after
Arthur C. Cope (1909–1966).
+
(a) Cope elimination
(b) Ester pyrolysis
..
..
..
..
..
..
–
+
CH
3
COOH
(67%)
N
O
CH
3
CH
3
CH
2
CH
2
H
2
CCH
3
OH(CH
3
)
2
N
..
..
O
O
..
..
O
..
..
O
..
..
575 ⬚C
+
WEB 3D
18.14 Special Topic: A Family of Concerted
Rearrangements of Acyl Compounds
In this section,we take up a few rearrangement reactions of some new kinds of mol-
ecules related to acyl compounds.The mechanisms are not always fully worked out,
but the reactions are of interest and are often synthetically useful.
18.14 Special Topic: A Family of Concerted Rearrangements of Acyl Compounds 915
O
R
Acid chloride
C
..
..
..
N
..
Cl
..
..
..
O
R
C
..
..
CH
..
N
Diazomethane
Diazo ketone
+
N
+
CH
2
N
..
–
–
FIGURE 18.63 Diazo ketone
formation.
The mechanism of this reaction is only slightly more complicated than the usual
reaction of nucleophiles with acid chlorides (p. 854). Diazo compounds are nucleo-
philes at carbon and add to the carbonyl group of the reactive acid chloride.Chloride
ion is expelled in the elimination phase of this normal addition–elimination process.
In the only new step of the reaction, chloride removes the newly acidic hydrogen to
give the diazo ketone (Fig. 18.64).This hydrogen is acidic because the conjugate base
is stabilized by resonance.
18.14a Diazo Ketones: The Wolff Rearrangement When an acid chloride
is allowed to react with a diazo compound, the result is formation of a diazo ketone
(Fig. 18.63).
O
R
C
H
H
..
..
O
R
C
..
..
Cl
..
..
..
..
Cl
..
..
..
Diazo ketone
N
N
..
N
..
N
+
+
CH
2
N
..
–
–
–
–
(–)
(–)
C
O
RN
C
..
..
CH
R
C
C
H
N
+
+
N
H
..
O
..
..
..
addition
elimination
deprotonation
Cl
..
..
..
..
..
Cl
..
..
..
H
+
FIGURE 18.64 The mechanism of
diazo ketone formation.
Diazo compounds are generally rather dangerous. They are not only poisonous
but explosive as well. The resonance-stabilized diazo ketones are far less explosive
than simple diazo compounds, but they must still be treated with the greatest respect.
PROBLEM 18.26 Draw resonance forms for a diazo ketone.
Like other diazo compounds, diazo ketones are sensitive to both heat and light.
Upon exposure to either, nitrogen is lost and a carbene intermediate (p. 431), in this
case, a ketocarbene, is formed (Fig. 18.65).
O
R
C
..
..
A ketocarbene
Addition
to alkenes
Insertion into
C
H bonds
N
..
..
N
or hν
..
N
+
CH
–
O
R
C
..
..
..
CH
O
R
C
..
..
..
CH
Keto carbene
O
R
C
..
..
..
N
+
⌬
H
C
CH
C HC
C
H
R O
..
..
FIGURE 18.65 Diazo ketones
decompose to give ketocarbenes on
heating or photolysis. The reactions
of ketocarbenes include additions to
π systems and carbon–hydrogen
insertion.
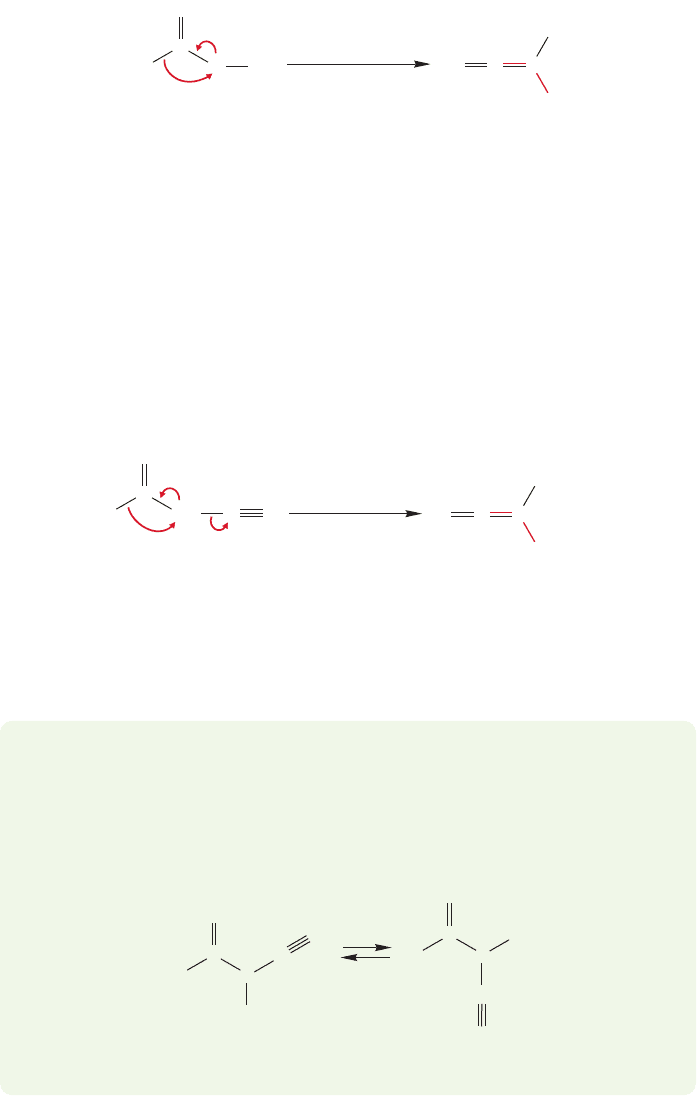
916 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
Ketocarbenes are capable of the usual carbene reactions, addition to multiple
bonds and insertion into carbon–hydrogen bonds chief among them (Fig. 18.65).
In addition, it is possible for ketocarbenes to undergo an intramolecular
carbon–carbon insertion reaction. This reaction is often catalyzed by silver,
and involves a migration of the R group (Fig. 18.66). The product of this reaction
is a ketene. This reaction is known as the Wolff rearrangement, after Ludwig
Wolff (1857–1919; remember the Wolff–Kishner reduction, p. 645? Same Wolff).
O
R
C
..
..
Wolff
rearrangement
..
H
C
OC
R
A ketene
H
C
..
..
FIGURE 18.66 Intramolecular ketene formation from a
ketocarbene, through a migration of an R group.
O
R
C
..
..
....
NN
CH
–
N
2
loss of N
2
as
R migrates
OC
R
A ketene
H
C
..
..
+
+
FIGURE 18.67 In the Wolff rearrangement, the bond can
break as the nitrogen departs. Perhaps there is no ketocarbene
intermediate.
C
O
R
The mechanistic complication in a Wolff rearrangement comes from the notion
that it may not be the carbene, but the diazo compound itself that gives the ketene.
The carbene is not necessarily involved (Fig. 18.67). The question isn’t completely
sorted out yet, but the best evidence currently favors at least some direct formation
of the ketene from most diazo ketones. However the evidence doesn’t exclude the
involvement of the carbene in all cases.
WORKED PROBLEM 18.27 Much experimentation indicates that only one of the
two possible stereoisomers of a diazo ketone can give ketene directly. What are
the two stereoisomers of the diazo ketone?
ANSWER There are s-cis and s-trans isomers of diazo ketones.
+
–
C
R
C
N
N
H
..
O
..
..
..
s-cis
+
–
C
R
C
H
N
N
..
..
O
..
..
s-trans
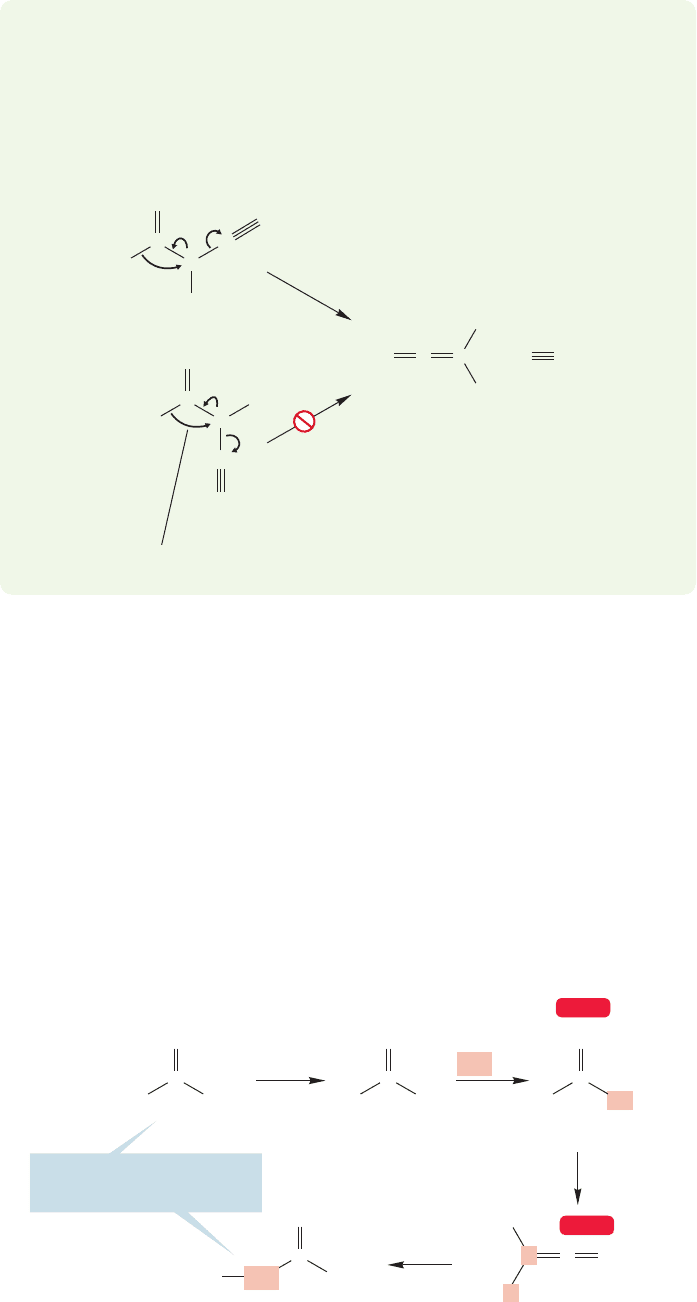
18.14 Special Topic: A Family of Concerted Rearrangements of Acyl Compounds 917
If the mechanistic questions still are not completely resolved, the synthetic
applications certainly are. The Wolff rearrangement generates a ketene in the
absence of nucleophiles, and therefore the ketene can be isolated in this reaction.
If the product ketene is subsequently hydrolyzed to the carboxylic acid, the over-
all sequence is called the Arndt–Eistert reaction after Fritz Arndt (1885–1969)
and Bernd Eistert (1902–1978). It constitutes a most useful chain-lengthening
reaction (Fig. 18.68). The acid chloride that was used at the outset (Fig. 18.63)
must come from a carboxylic acid, because that is the only way we have for mak-
ing an acid chloride. So the overall process starting with the original acid is as fol-
lows: make the acid chloride, convert the acid chloride into the diazo ketone,
rearrange the ketone to the ketene, and hydrolyze the ketene to give the new car-
boxylic acid.
+
–
C
R
C
N
N
H
..
O
..
..
..
s-cis
–
C
R
C
..
O
..
..
OCC
H
H
R
..
..
s-trans
Frontside S
N
2—No, No, No
+
N
N
..
N
..
N
..
Acid
New acid
Acid chloride Diazo ketone
R
C
Cl
..
..
SOCl
2
CH
2
N
2
⌬/Ag
CHN
2
OCC
R
H
Ketene
..
..
+
N
2
O
..
..
..
C
OH
..
..
O
..
..
..
R
C
O
..
..
..
..
H
2
O
R
CH
2
Compare these two acids—
a CH
2
has been added
O
R
C
..
OH
..
..
WEB 3D
WEB 3D
FIGURE 18.68 The Arndt–Eistert
synthesis adds a methylene group
to a carboxylic acid.
WORKED PROBLEM 18.28 Explain why only one of the isomers in the previous
problem can rearrange easily to a ketene.
ANSWER The s-cis isomer is set up for migration of R with displacement from
the rear in an intramolecular S
N
2 reaction. By contrast, the s-trans isomer would
have to do a frontside S
N
2 for R to migrate as the nitrogen leaves.
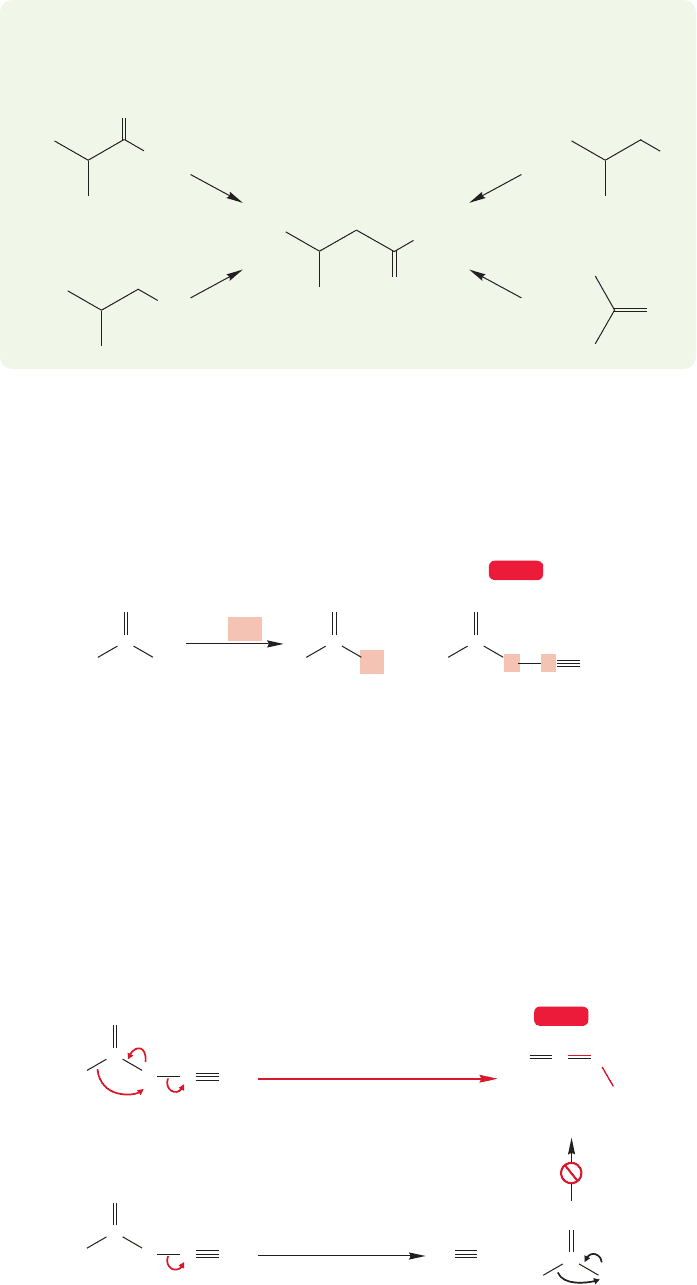
918 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
(a)
OH
O
3-Methylbutanoic acid
OH
O
(b)
OH
(c)
(d)
I
18.14b Acyl Azides: The Curtius Rearrangement Acyl azides are related
to diazo ketones, and can be made by reaction of acid chlorides with the azide ion,
N
3
(Fig. 18.69).
R
An acyl azide
C
R
=
C
O
..
..
O
..
..
R
C
O
..
..
Cl
..
..
..
Na
+
N
3
–
–
+
N
3
N N
..
..
N
..
WEB 3D
FIGURE 18.69 Acid chlorides react
with azide ion (
N
3
) to give acyl
azides.
Like diazoketones, acyl azides lose nitrogen when heated or irradiated.The end
result is an isocyanate, formed either directly from the acyl azide, in a reaction called
the Curtius rearrangement after Theodore Curtius (1857–1928), or by intramol-
ecular rearrangement of a nitrene, which is the nitrogen analogue of a carbene
(Fig. 18.70). For most acyl azides,it is known that the isocyanate is formed by direct
rearrangement of the acyl compound and that the nitrene, though also formed from
the azide, is not involved in isocyanate production.
R
C
O
..
..
N
..
..
..
..
–
N
..
R
C
O
..
..
+
NN
N
..
migration of R as N
2
is lost
An isocyanate
A nitrene
loss of N
2
OC
R
..
..
+
N
..
..
N
..
..
..
–
R
C
O
..
..
+
NN
N
..
WEB 3D
FIGURE 18.70 The Curtius
rearrangement leads to isocyanates.
The mechanism usually involves a
concerted, one step rearrangement.
PROBLEM 18.29 Devise a synthetic scheme for converting each of the starting
materials shown into 3-methylbutanoic acid.
