Middleton W.M. (ed.) Reference Data for Engineers: Radio, Electronics, Computer and Communications
Подождите немного. Документ загружается.

15-30
REFERENCE
DATA
FOR ENGINEERS
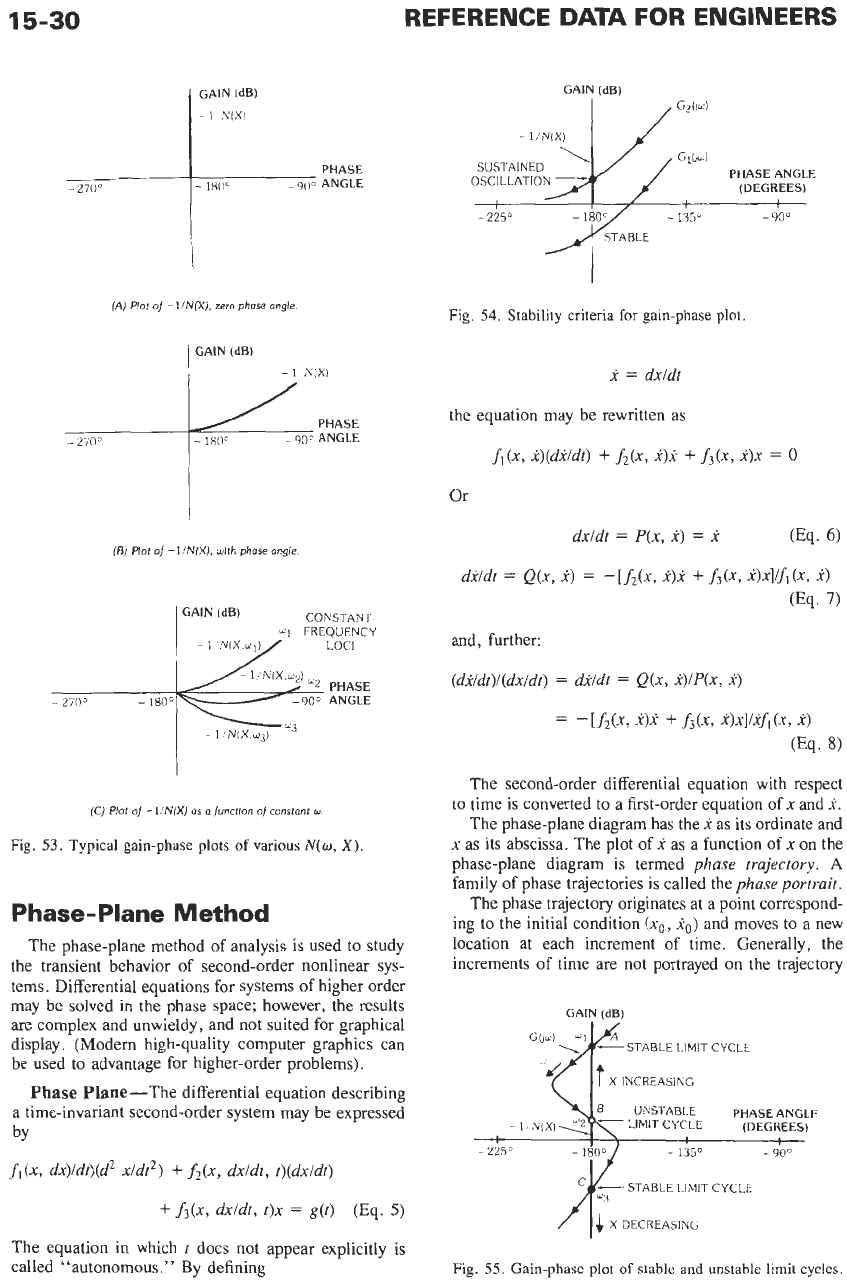
(A)
Plot
of
-
l/N(X),
zero
phase
angle
i
-
1
NiXi
(E)
Plot
of
-l/N(Xj,
with
phose
ongle
CONSTANT-
ai
FREQUENCY
GAIN
(dB)
-
270’
-
1800
-90”
ANGLE
(C)
Plot
of
-l/N(Xj
os
a
junctlon
of
constant
w.
Fig.
53.
Typical gain-phase plots
of
various
N(w,
X).
Phase-Plane Method
The phase-plane method of analysis is used to study
the transient behavior of second-order nonlinear sys-
tems. Differential equations for systems of higher order
may
be solved
in
the
phase
space; however,
the
results
are complex and unwieldy, and not suited for graphical
display. (Modern high-quality computer graphics can
be used
to
advantage for higher-order problems).
Phase
Plane-The differential equation describing
a time-invariant second-order system may be expressed
by
fi(x,
dn)ldt)(d2 x/dt2)
+
f2(x,
dxldt, t)(dxldt)
+
&(x,
dxldt, t)x
=
g(t)
(Eq.
5)
The equation in which
t
does not appear explicitly
is
called “autonomous.” By defining
GAIN
(dB)
G2bl
l/N(X)
Gib4
PHASE ANGLE
SUSTAINED
(DEGREES)
OSCILLATION
?-
-
1800
-
135O
Fig.
54.
Stability criteria for gain-phase plot.
x
=
dxldi
the equation may be rewritten as
fi(~,
I)(di/dt)
+
f2(~,
X)X
+
fj(~,
i)~
=
0
Or
dxidt
=
P(x,
R)
=
x
(Eq.
6)
(Eq.
7)
diidt
=
Q(x,
R)
=
-[f2(~,
X).t
+
&(x,
i)~]ifi(~,
i)
and. further:
(dxldt)l(dxldt)
=
dddt
=
Q(x,
i)/P(x,
x)
The second-order differential equation with respect
to time is converted to a first-order equation of
x
and
1.
The phase-plane diagram has the
x
as its ordinate and
x
as its abscissa. The plot of
3
as a function of
n
on the
phase-plane diagram
is
termed
phase trajectory.
A
family of phase trajectories is called the
phase portrait.
The phase trajectory originates at a point correspond-
ing to the initial condition
(xg
,
&)
and moves to a new
location at each increment of time. Generally, the
increments of time are not portrayed on the trajectory
GAIN
(dB)
UNSTABLE
PHASE ANGLE
-
90‘
~
22.50
-
180°
-
135’
I
LIMIT CYCLE
X
DECREASING
Fig.
55.
Gain-phase plot
of
stable and unstable limit cycles.
FEEDBACK CONTROL SYSTEMS
15-31
GAIN(dB)
PHASE ANGLE
(DEGREES)
-
2250
-
135O
-
900
SUSTAINED
-
OSCILLATION
Fig.
56.
Typical gain-phase
plot
of
C(jo)
and
-[l/N(w,
X)]
as
a
function
of
w.
and must be obtained by other means. If the value of
time at each point on the trajectory is obtained, the time
response of
x(t)
and
x(t)
can be plotted. The phase
trajectory has a definite direction associated with time.
When
1
is positive the trajectory moves from left to
right, and for negative values of
1
all paths move from
right to left. If the trajectory approaches the origin or
some finite point on the phase plane as time goes to
30,
the system is stable. If the trajectory goes to
30
with
time, the system is unstable. If the trajectory approach-
es an enclosed path in the phase plane, the system has
sustained oscillation. The enclosed path is called the
limit cycle.
Construction
of
the Phase Portrait, Method
of
Isoclines-The slope of
dildx
of Eq.
8
is simply the
slope
of
the trajectory in the phase plane. The locus of
constants
dx/dx
is termed an
isocline
corresponding to
the slope
a;
that is
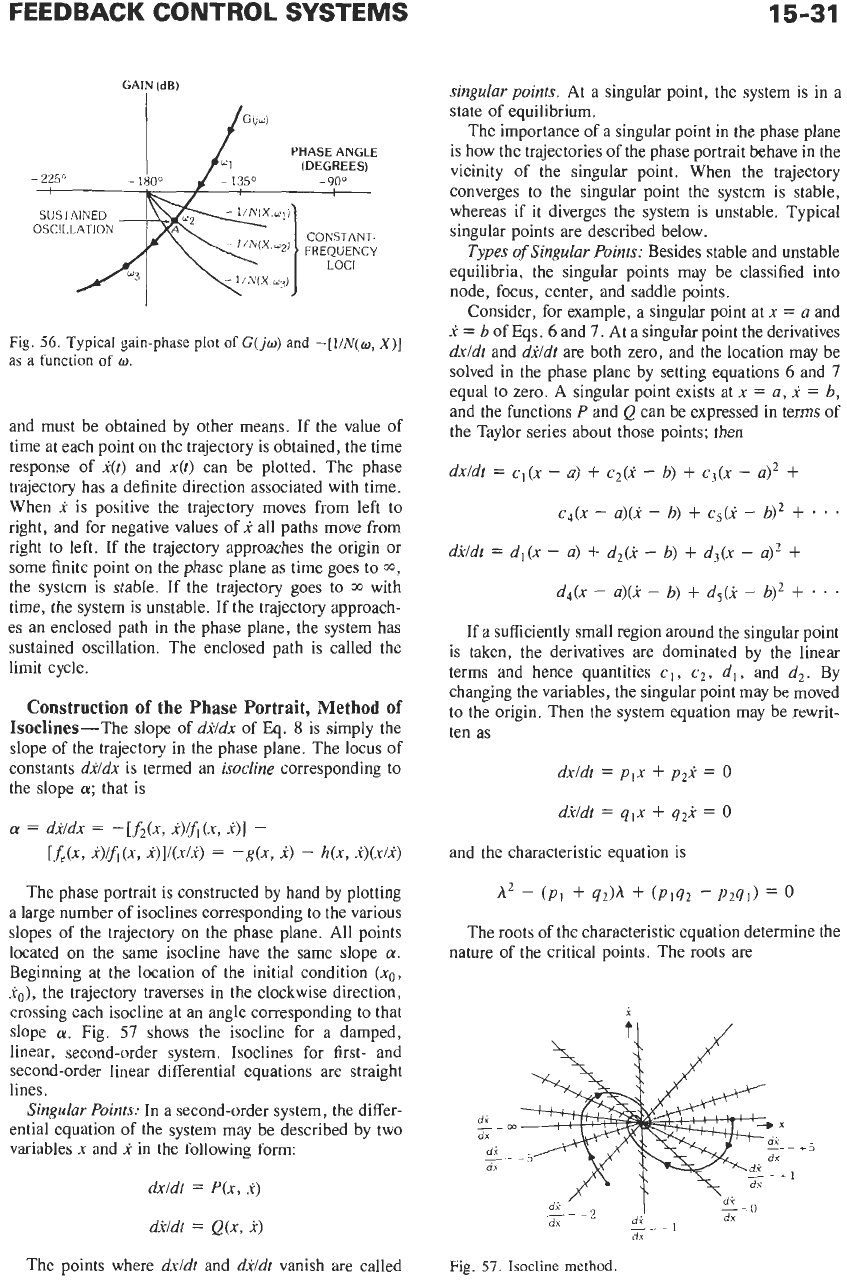
The phase portrait is constructed by hand by plotting
a large number
of
isoclines corresponding to the various
slopes of the trajectory on the phase plane. All points
located on the same isocline have the same slope
a.
Beginning at the location
of
the initial condition
(xo,
xo),
the trajectory traverses in the clockwise direction,
crossing each isocline at an angle corresponding to that
slope
a.
Fig.
57
shows the isocline for a damped,
linear, second-order system. Isoclines for first- and
second-order linear differential equations are straight
lines.
Singular
Points:
In a second-order system, the differ-
ential equation of the system may be described by two
variables
x
and
1
in the following form:
dxldt
=
P(x,
x)
dxldt
=
Q(x,
x)
The points where
dxldt
and
dildt
vanish are called
singular points.
At a singular point, the system is in a
state of equilibrium.
The importance of a singular point in the phase plane
is how the trajectories of the phase portrait behave in the
vicinity of the singular point. When the trajectory
converges to the singular point the system is stable,
whereas if it diverges the system is unstable. Typical
singular points are described below.
Types
of
Singular
Points:
Besides stable and unstable
equilibria, the singular points may be classified into
node, focus, center, and saddle points.
Consider, for example, a singular point at
x
=
u
and
x
=
b
of
Eqs.
6
and
7.
At a singular point the derivatives
dxldt
and
dxldt
are both zero, and the location may be
solved in the phase plane by setting equations
6
and
7
equal to zero. A singular point exists at
x
=
a,
i
=
b,
and the functions
P
and
Q
can be expressed in terms of
the Taylor series about those points; then
dxldt
=
cI(x
-
a)
+
c~(X
-
b)
+
C~(X
-
a)*
t
c,(x
-
a)(i
-
b)
+
C5(i
-
by
+
*
’
.
dildt
=
dj(x
-
a)
+
d2(i
-
b)
+
d3(x
-
a)’
+
d,(x
-
u)(X
-
b)
+
d5(X
-
b)’
+
.
*
.
If a sufficiently small region around the singular point
is taken, the derivatives are dominated by the linear
terms and hence quantities
c,
,
c2,
d,
,
and
d2.
By
changing the variables, the singular point may be moved
to the origin. Then the system equation may be rewrit-
ten as
and the characteristic equation is
A*
-
(PI
+
42P
+
(PI42
-
P241)
=
0
The roots of the characteristic equation determine the
nature of the critical points. The roots are
x
dl
dx
-=a
di
dr
_=
dx
Fig.
57.
Isocline
method.
15-32
REFERENCE
DATA
FOR
ENGINEERS
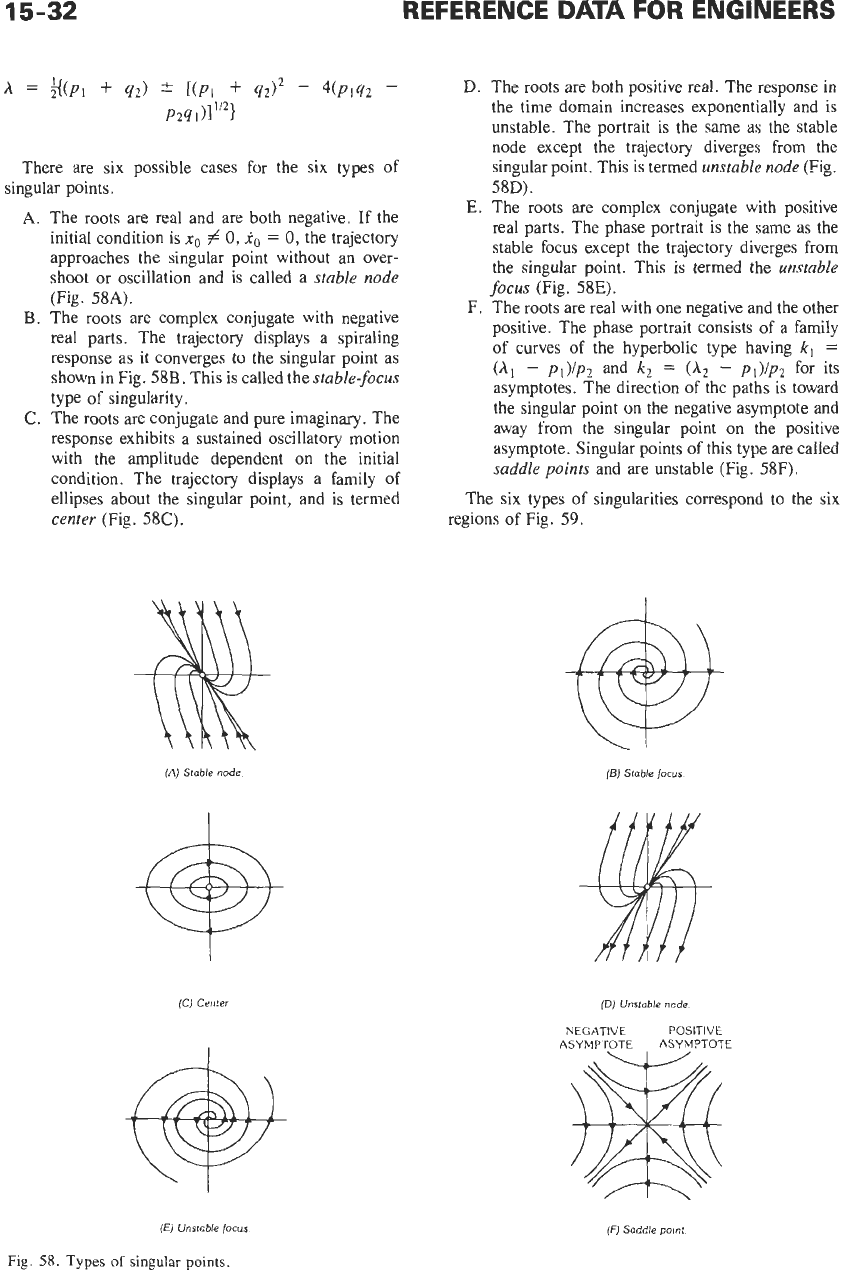
There are six possible cases for the six types of
A.
The roots are real and are both negative.
If
the
initial condition is
xo
# 0,
1,
=
0,
the trajectory
approaches the singular point without an over-
shoot or oscillation and
is
called a
stable node
(Fig.
58A).
B.
The roots are complex conjugate with negative
real parts. The trajectory displays
a
spiraling
response as it converges to the singular point as
shown in Fig.
58B.
This is called the
stable-focus
type
of
singularity.
C.
The roots are conjugate and pure imaginary. The
response exhibits a sustained oscillatory motion
with the amplitude dependent on the initial
condition. The trajectory displays a family
of
ellipses about the singular point, and is termed
center
(Fig.
58C).
singular points.
(A)
Stable
node
(C)
Center
D.
The roots are both positive real. The response in
the time domain increases exponentially and
is
unstable. The portrait is the same
as
the stable
node except the trajectory diverges from the
singular point. This is termed
unstable node
(Fig.
58D).
E.
The roots are complex conjugate with positive
real parts. The phase portrait is the same
as
the
stable focus except the trajectory diverges from
the singular point. This is termed the
unstable
focus
(Fig.
%E).
F.
The roots are real with one negative and the other
positive. The phase portrait consists of a family
of curves
of
the hyperbolic type having
k,
=
(A,
-
pI)/p2
and
k,
=
(A2
-
p1)/p2
for its
asymptotes. The direction of the paths is toward
the singular point on the negative asymptote and
away from the singular point on the positive
asymptote. Singular points of this type are called
saddle points
and are unstable (Fig.
58F).
The six types of singularities correspond to the six
regions of Fig.
59.
(E)
Stable
focus.
(D)
Unstoble
node
NEGATIVE POSITIVE
ASYMPTOTE ASYMPTOTE
(E)
Unstable
focus
Fig.
58.
Types
of
singular points.
(F)
Saddle
point.
FEEDBACK CONTROL SYSTEMS
15-33
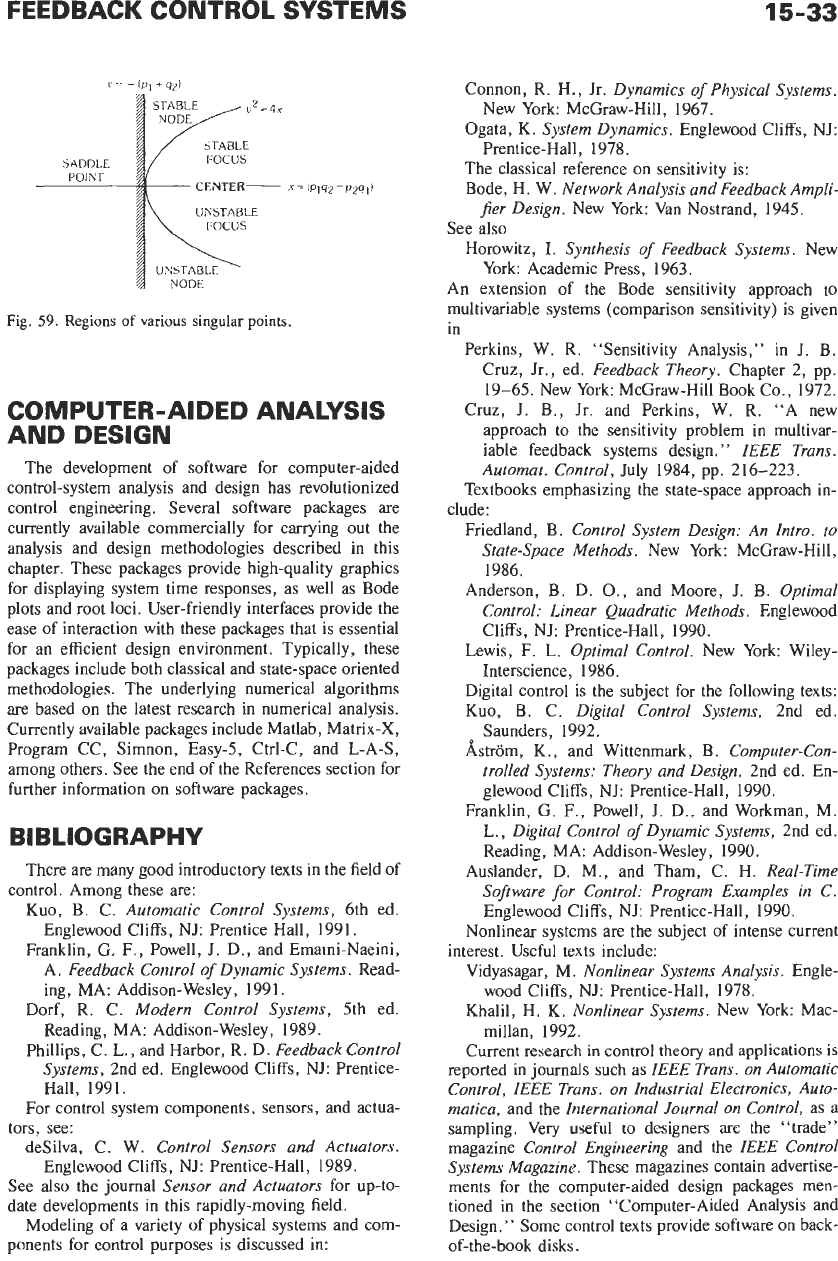
c=
-
ip,
+q2)
STABLE
SADDLE FOCUS
POINT
UNSTABLE
FOCUS
UNSTABLE
Fig.
59.
Regions
of
various singular points.
COMPUTER-AIDED ANALYSIS
AND DESIGN
The development of software for computer-aided
control-system analysis and design has revolutionized
control engineering. Several software packages are
currently available commercially for carrying out the
analysis and design methodologies described in this
chapter. These packages provide high-quality graphics
for displaying system time responses, as well as Bode
plots and root loci. User-friendly interfaces provide the
ease of interaction with these packages that is essential
for an efficient design environment. Typically, these
packages include both classical and state-space oriented
methodologies. The underlying numerical algorithms
are based
on
the latest research in numerical analysis.
Currently available packages include Matlab, Matrix-X,
Program CC, Simnon, Easy-5, Ctri-C, and L-A-S,
among others. See the end of the References section for
further information on software packages.
BIBLIOGRAPHY
There are many good introductory texts in the field of
Kuo, B. C.
Automatic Control Systems,
6th ed.
Englewood Cliffs, NJ: Prentice Hall, 1991.
Franklin, G.
F.,
Powell, J.
D.,
and Emami-Naeini,
A.
Feedback Control
of
Dynamic Systems.
Read-
ing, MA: Addison-Wesley, 1991.
Dorf,
R.
C.
Modern Control Systems,
5th ed.
Reading, MA: Addison-Wesley, 1989.
Phillips, C.
L.,
and Harbor, R. D.
Feedback Control
Systems,
2nd ed. Englewood Cliffs, NJ: Prentice-
Hall, 1991.
For control system components, sensors, and actua-
desilva, C. W.
Control Sensors and Actuators.
Englewood Cliffs, NJ: Prentice-Hall, 1989.
See also the journal
Sensor and Actuators
for up-to-
date developments in this rapidly-moving field.
Modeling of a variety of physical systems and com-
ponents for control purposes is discussed in:
control. Among these are:
tors, see:
Connon,
R.
H., Jr.
Dynamics of Physical Systems.
Ogata, K.
System Dynamics.
Englewood Cliffs, NJ:
The classical reference
on
sensitivity is:
Bode, H. W.
Network Analysis and Feedback Ampli-
New York: McGraw-Hill, 1967.
Prentice-Hall, 1978.
fier Design.
New York: Van Nostrand, 1945.
See also
Horowitz,
I.
Synthesis
of
Feedback Systems.
New
An extension of the Bode sensitivity approach to
multivariable systems (comparison sensitivity) is given
in
Perkins, W.
R.
“Sensitivity Analysis,” in J. B.
Cruz, Jr., ed.
Feedback Theory.
Chapter 2, pp.
19-65. New York: McGraw-Hill Book Co., 1972.
Cruz, J. B., Jr. and Perkins, W. R. “A new
approach to the sensitivity problem in multivar-
iable feedback systems design.”
IEEE Trans.
Automat. Control,
July 1984, pp. 216-223.
Textbooks emphasizing the state-space approach in-
Friedland, B.
Control System Design: An Intro. to
State-Space Methods.
New York: McGraw-Hill,
1986.
Anderson, B. D. O., and Moore, J. B.
Optimal
Control: Linear Quadratic Methods.
Englewood
Cliffs, NJ: Prentice-Hall, 1990.
Lewis,
F.
L.
Optimal Control.
New York: Wiley-
Interscience, 1986.
Digital control is the subject for the following texts:
Kuo, B. C.
Digital Control Systems,
2nd ed.
Saunders, 1992.
krom, K., and Wittenmark, B.
Computer-Con-
trolled Systems: Theory and Design,
2nd ed. En-
glewood Cliffs, NJ: Prentice-Hall, 1990.
Franklin,
G.
F.,
Powell, J.
D.,
and Workman, M.
L.,
Digital Control of Dynamic Systems,
2nd ed.
Reading, MA: Addison-Wesley, 1990.
Auslander, D. M., and Tham, C. H.
Real-Time
Software
for
Control: Program Examples in C.
Englewood Cliffs, NJ: Prentice-Hall, 1990.
Nonlinear systems are the subject of intense current
Vidyasagar, M.
Nonlinear Systems Analysis.
Engle-
Khalil, H. K.
Nonlinear Systems.
New York: Mac-
Current research in control theory and applications
is
reported in journals such
as
IEEE
Trans. on Automatic
Control, IEEE Trans.
on
Industrial Electronics, Auto-
matica,
and the
International Journal on Control,
as a
sampling. Very useful
to
designers are the “trade”
magazine
Control Engineering
and the
IEEE Control
Systems Magazine.
These magazines contain advertise-
ments for the computer-aided design packages men-
tioned in the section “Computer-Aided Analysis and
Design.” Some control texts provide software on back-
of-the-book disks.
York: Academic Press, 1963.
clude:
interest. Useful texts include:
wood Cliffs, NJ: Prentice-Hall, 1978.
millan, 1992.

16
Electron
Tubes
Marvin Chodorow and Robert Symons
Revised by David Abe, Richard Abrams, Bruce Danly, Henry Freund, Kevin Jensen,
Baruch Levush, Robert Myers, Robert Parker, Jonathan Shaw, Arnold Shih, Joan Yater
Electron Emission
16-3
Thermionic Emission
Secondary Emission
Field Emission
Macroscopic Current Density
Field Emitter Arrays
Current-Voltage Relationship, Transconductance, and Modulation
Protective Resistance
Field Emission Displays
Photocathodes
Electrode Dissipation
16-16
Radiation Cooling
Water Cooling
Forced-
Air
Cooling
Evaporative Cooling
Conduction Cooling
Grid Temperature
Noise
in
Tubes
16-1
8
Shot Effect
Partition Noise
Flicker Effect
Collision Ionization
Induced Noise
Miscellaneous Noise
Microwave Tubes
16-1

16-2
REFERENCE
DATA
FOR ENGINEERS
Low-, Medium-, and High-Frequency Tubes
16-19
Coefficients
Materials and Structures
16-21
Cathodes
Grids
Anodes
Getters
Tube Geometry
Microwave Tubes
16-23
Terminology
Recent Trends
Linear-Beam Tubes
Crossed-Field Tubes
Cyclotron Resonance Microwave Tubes
Free-Electron Lasers
16-39
Gas Tubes
16-41
Characteristics of Gas Tubes
Power Applications of Gas Tubes
Microwave Applications of Gas Tubes
Light-Sensing and Light-Emitting Tubes
Radiometry and Photometry
Flux Units
Optical Imaging
16-38
16-45
Typical Approximate Illumination Values at the Surface of the Earth
Typical Approximate Brightness Values
Light-Sensing Tubes
16-48
Image Tubes and Image lntensifiers
Gas Photodiodes
Image Orthicons
Vidicons
Variations of the Vidicon
Light-Emitting Tubes
16-55
Cathode-Ray Tubes
Storage Cathode-Ray Tubes
Bistable Storage Tube
ELECTRON EMISSION
All electron tubes“ depend for their operation on the
flow of electrons within the tube, through either high
vacuum or
an
ionized gas. The electrons are emitted
from a cathode surface as a result of one of four pro-
cesses that are distinguished on the basis of
the
mecha-
nism by which the electrons are enabled to leave the
surface. These processes are elevated temperature
(thermionic or primary emission); bombardment by
other particles, generally electrons (secondary emis-
sion); the action of a high electric field (field emis-
sion); or the incidence of photons (photoemission).
Therm
ionic
Em
ission
Thermionic emission originates from the thermally
excited electrons that have sufficient kinetic energy to
overcome the vacuum barrier. The sum of all these ther-
mal electrons gives rise to the Richardson-Dushman
equation, which
is
J
AT^
exp(-+/kT) (Eq.
1)
where
J
is emission density in amperes/cm2,
A
is 120 amperes/cm2,
+
is work function in electron volts (eV),
T
is temperature in kelvins
(K),
and
k,
the Boltzmann constant, is 8.6164
x
lob5
eV
K-l.
Emission density is
an
exponential function of the
work function. Between
1000
K
and 2000
K,
a
decrease in the work function of 0.2 to 0.4 eV causes
an
order of magnitude increase in the emission density.
The work function of commonly used thermionic cath-
odes varies from 4.6 eV (tungsten) to 1.5 eV (oxide-
coated cathodes).
The first thermionic cathodes used in quantity were
made from pure tungsten
(W).
Because
of
the high
work function, a very high temperature is required to
provide a reasonable emission density. For example,
Eq.
1
indicates that an emission density of
0.3
A/cm2
requires a temperature of 2150°C (or 2423
K).
To reduce the operating temperature, thoria (tho-
rium oxide) was mixed with
W.
Thorium would diffuse
to the surface of
W
and lower the work function. The
cathode surface was sometimes carburized to reduce
the thorium evaporation, extending the operating life.
The resulting surface has
a
work function of
-
3.2
eV.?
For comparison with pure tungsten, the emission den-
*
J.
W.
Gewartowski
and
H.
A.
Watson,
Principles
Of
Electron Tubes
(Princeton, NJ: Van Nostrand, 1965).
K.
R.
Spangenberg,
Vacuum Tubes
(New York: McGraw-Hill
Book
Co., 1948).
A.
H.
W. Beck,
Thermionic Valves, Their Theory
and Design
(London:
Cambridge University
Press,
1953).
Standards on Electron Tubes: Definitions
of
Terms
(New
York:
Institute
of
Radio Engineers, 1950).
t
Reference
1.
sity of 0.3 A/ cm2 requires 1950°C (2223
K),
a 200°C
reduction in temperature.
A
significant reduction in work function was real-
ized
in
oxide-coated cathodes. The oxide-coated cath-
odes consist of an alkaline-earth-oxide coating on a
metal substrate, which is usually nickel (Ni). A stan-
dard ASTM (American Society for Testing and Materi-
als) mixture of the oxide coating is 49? 44, and
7
atomic percent of Ba, Sr, and Ca oxide, respectively.
The substrate can be made of either active or passive
Ni, depending on whether or not it contains reducing
agents. A typical active Ni contains 4%
W
in the bulk.
The work function of a fully activated oxide-coated
cathode is 1.85 eV and
1.5
eV on passive and active Ni
substrates, respectively.$
The initial alkaline earths are
in
carbonate form and
mixed by an organic binder. The carbonates and binder
are either sprayed onto the substrate or they are cut and
transferred from a self-supporting sheet, which is
known as a “Sarong” coating. Activation of an oxide-
coated cathode requires several hours of outgassing. A
copious quantity of hydrocarbon gases is released ini-
tially from the decomposition of the organic binder,
and then a large amount of CO, is released from the
decomposition of the carbonates into the respective
oxides.
It
is advisable to raise the cathode temperature
gradually during the carbonate conversion to avoid
CO, overpressure, which could cause melting of the
oxide coating. On some oxide-coated cathodes, high
work function patches were observed
to
form, which
were identified as the formation of CaO slags on the
surface caused by BaO/BaCO, eutectic melting during
the conversion process.§
The activation and poisoning processes of an oxide-
coated cathode are explained by a semiconductor
model.” The oxygen vacancies in the oxide lattice act
as electron donors. During activation, the reducing
agents, such as
W
in the Ni substrate, react and bind
with the oxygen atoms in the oxides and generate oxy-
gen vacancies. The creation of these donors shifts the
Fermi level up from
the
intrinsic mid-band gap to near
the bottom of the conduction band, and consequently,
the work function is lowered. Thermal excitation lifts
some of the electrons
in
the donors to the conduction
band, and the mobile electrons are needed in the elec-
tron emission process. Cathode poisoning by oxidizing
gases such as
0,,
H,O,
and CO, occurs through filling
the oxygen vacancies and shifting the Fermi level back
toward
the
mid-band gap. Sulfur, which is present on
the surface of many metals, can have a detrimental
effect on the oxide-coated cathodes since
S
can
also
fill the oxygen vacancies. The well-known 10-volt
slump occurs when
S
is transferred from the metal
grids
to
the oxide coating through electron-stimulated
$
Reference
2.
§
Reference
3.
‘I
Reference
4.
REFERENCE
DATA
FOR ENGINEERS
desorption. An oxide-coated cathode, once poisoned
by
S,
cannot be reactivated.*
The major limitations of oxide-coated cathodes
are
low dc emission and susceptibility to damage. Because
moderate electrical conductivity is inherent to semicon-
ductive alkaline earth oxides, the dc emission of the
oxide-coated cathodes is limited to less than
1
A/cm2.
Pulsed emission densities are dependent on pulse
length and can be as high as tens of A/cm2.t After the
conversion from carbonates to the oxides, it is impera-
tive to maintain a good vacuum since reactivation after
exposure
to
oxidizing gases is often difficult.* Expo-
sure
to
atmosphere is always detrimental since the
coating expansion accompanying the hydroxide for-
mation would cause the oxide coating to peel.
Dispenser cathodes are now the most commonly
used high emission-density cathodes. Instead of a
thick oxide coating, only a mono-layer or less of Ba
and
0
covers the metal substrate of a dispenser cath-
ode. Since the cathode body is made of W metal, the
dc emission is no longer limited by the electrical con-
ductivity. During the operation of a dispenser cathode,
a fresh supply
of
active materials (Ba and
0)
is contin-
uously dispensed onto the emitting surface, replenish-
ing the loss due to evaporation. Consequently, the
dispenser cathodes are quite robust in comparison to
the oxide-coated cathodes. During the fabrication of a
dispenser cathode, W powder with an average grain
size of 4.5 pm is pressed
to
form the cathode body of a
W-matrix with 20% porosity (i.e.,
80%
density of W).
The pores of the W-matrix are then impregnated with a
melt of mixed calcined BaO/CaO/Al,O, drawn into
the pores by capillary action in an atmosphere of
dry
hydrogen. Consequently, dispenser cathodes are also
known
as impregnated cathodes or matrix cathodes.
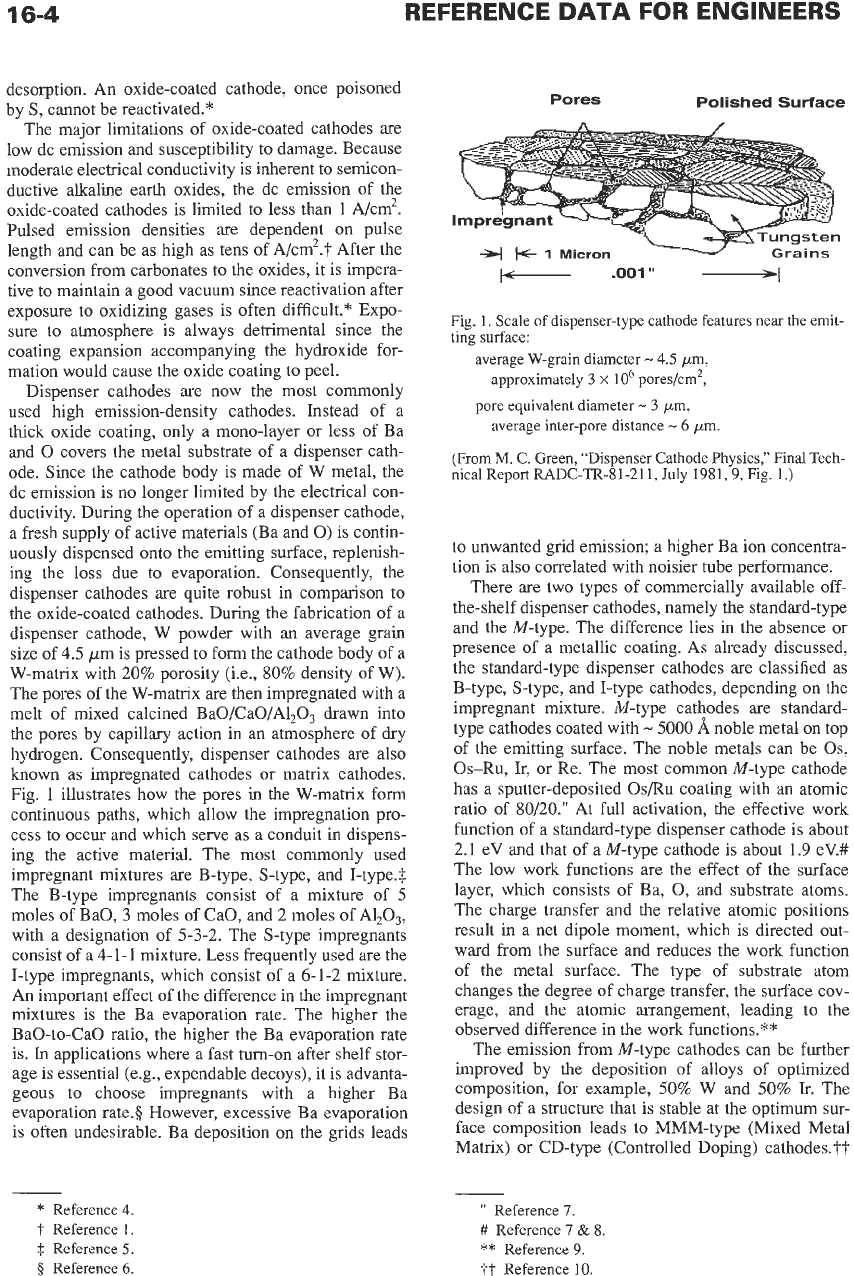
Fig.
1
illustrates how the pores in the W-matrix form
continuous paths, which allow the impregnation pro-
cess to occur and which serve as a conduit in dispens-
ing the active material. The most commonly used
impregnant mixtures are B-type, S-type, and I-type.$
The B-type impregnants consist of a mixture of
S
moles of BaO,
3
moles
of
CaO, and 2 moles
of
A1,0,,
with a designation of 5-3-2. The S-type impregnants
consist of a 4-1-1 mixture. Less frequently used are the
I-type impregnants, which consist
of
a 6-1-2 mixture.
An important effect
of
the difference
in
the impregnant
mixtures is the Ba evaporation rate. The higher the
BaO-to-CaO ratio, the higher the Ba evaporation rate
is.
In
applications where a fast turn-on after shelf stor-
age
is
essential (e.g., expendable decoys), it
is
advanta-
geous to choose impregnants with a higher Ba
evaporation rate.§ However, excessive Ba evaporation
is often undesirable.
Ba
deposition on the grids leads
Pores Polished
Surface
1
Micron
t-..
.001”
-1
Fig.
1.
Scale of dispenser-type cathode features
near
the emit-
ting surface:
average W-grain diameter
-
4.5
pm,
approximately
3
x
lo6
pores/cm2,
pore equivalent diameter
-
3
pm,
average inter-pore distance
-
6
pm.
(From
M.
C.
Green, “Dispenser Cathode Physics,” Final Tech-
nicalReport RADC-TR-81-211,
July 1981,9, Fig. 1.)
to unwanted grid emission; a higher Ba ion concentra-
tion is also correlated with noisier tube performance.
There are two types of commercially available off-
the-shelf dispenser cathodes, namely the standard-type
and the M-type. The difference lies in the absence or
presence of a metallic coating. As already discussed,
the standard-type dispenser cathodes are classified as
B-type, S-type, and I-type cathodes, depending on the
impregnant mixture. M-type caGodes
are
standard-
type cathodes coated with
-
5000
A noble metal on top
of the emitting surface. The noble metals can be
Os,
Os-Ru,
Ir,
or Re. The most common M-type cathode
has a sputter-deposited
Os/Ru
coating with an atomic
ratio of 80/20.” At full activation, the effective work
function of a standard-type dispenser cathode is about
2.1 eV and that of a M-type cathode is about
1.9
eV.#
The low work functions are the effect
of
the surface
layer, which consists of Ba,
0,
and substrate atoms.
The charge transfer and the relative atomic positions
result in a net dipole moment, which is directed out-
ward from the surface and reduces the work function
of the metal surface. The type of substrate atom
changes the degree of charge transfer, the surface cov-
erage, and the atomic arrangement, leading to the
observed difference in the work functions.**
The emission from M-type cathodes can be further
improved by the deposition of alloys
of
optimized
composition, for example,
50%
W and
SO%
Ir.
The
design of a structure that is stable at the optimum sur-
face composition leads to MMM-type (Mixed Metal
Matrix) or CD-type (Controlled Doping) cathodes.??
*
Reference
4.
t
Reference
1.
j:
Reference
5.
5
Reference
6.
”
Reference
7.
#
Reference
7
&
8.
**
Reference 9.
tt
Reference
10.
ELECTRON TUBES
The cathode body of a MMM-type cathode is a sin-
tered plug of
W
and noble metal (usually
Ir)
particles.
The surface coating of a CD-type cathode consists of
deposited multiple layers of alloy material, each of dif-
ferent composition. However, these types of cathodes
are no longer commercially available as off-the-shelf
items.
Scandate cathodes have been under study for many
years.
In
early years, their performance had not been
stable or reproducible. However, recent developments
at Philips Research Laboratories have demonstrated
consistent emission for several thousand hours in
diode tests from top-layer type scandate cathodes,
which were fabricated by depositing a layer of
SqO?
and W mixture on a S-type cathode. At 1O3O0C, emis-
sion densities of
100
A/cm2 and
400
A/cm2 have been
demonstrated for top-layer scandate cathodes prepared
by a mixed powder method* and laser ablation deposi-
tion,? respectively. These emission densities corre-
spond to effective work functions of
1.63
eV and
1.48
eV, respectively. Even in applications that require an
emission density lower than
100
A/cm2, the use of
scandate cathodes allows operation at a lower tempera-
ture, which will result in lower Ba evaporation and
longer life.
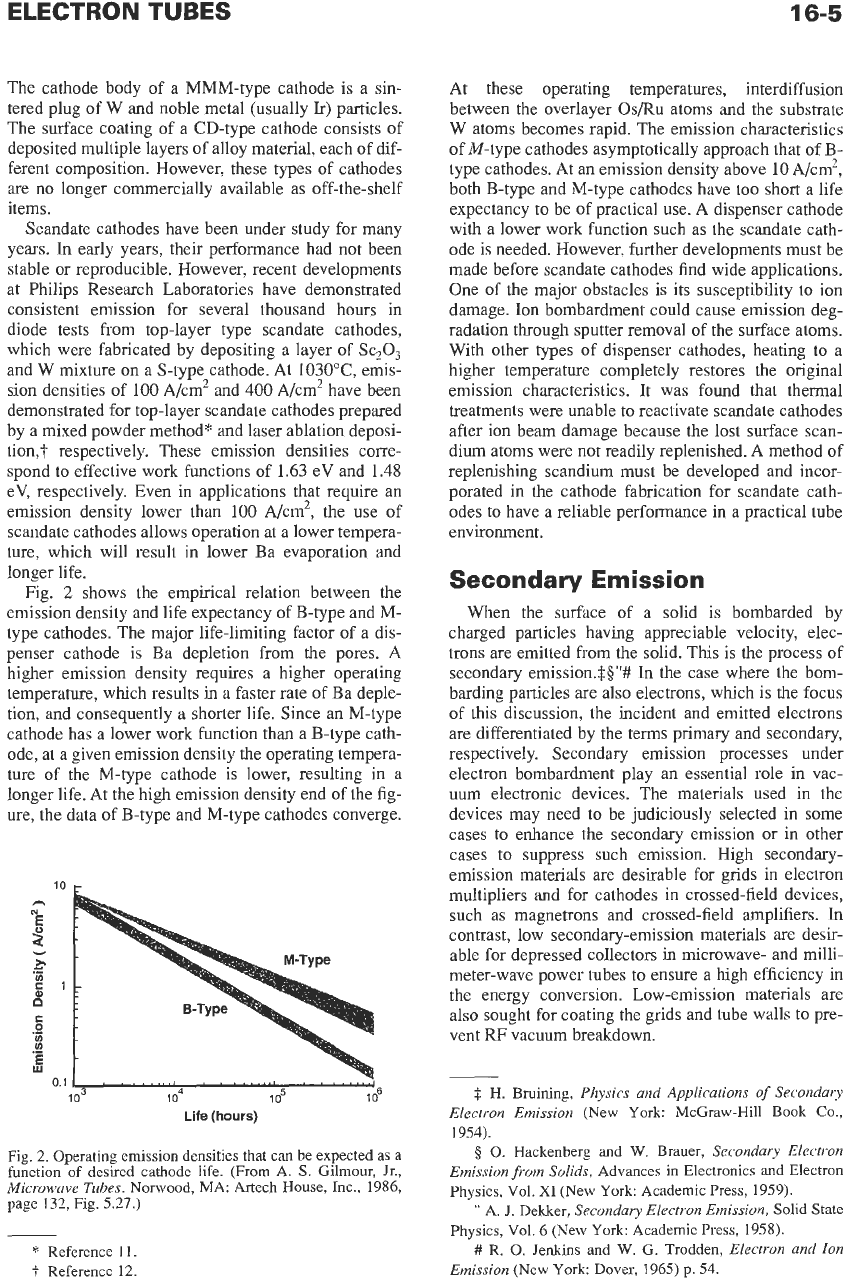
Fig.
2
shows the empirical relation between the
emission density and life expectancy of €3-type and
M-
type cathodes. The major life-limiting factor of a dis-
penser cathode is Ea depletion from the pores.
A
higher emission density requires a higher operating
temperature, which results in a faster rate of Ea deple-
tion, and consequently a shorter life. Since
an
M-type
cathode has
a
lower work function than a B-type cath-
ode, at a given emission density the operating tempera-
ture of the M-type cathode is lower, resulting
in
a
longer life. At the high emission density end of the fig-
ure, the data of B-type and M-type cathodes converge.
h
"E
.-
b
?I
d
2
-
C
Lo
.-
.-
E
w
0.1
1
o3
1
o4
1
o5
1
06
Life
(hours)
Fig.
2.
Operating emission densities that can be expected
as
a
function
of
desired cathode life. (From
A.
S. Gilmour, Jr.,
Microwave
Tubes.
Norwood,
MA:
Artech House, Inc., 1986,
page 132, Fig. 5.27.)
*
Reference
11.
t
Reference 12.
At these operating temperatures, interdiffusion
between the overlayer
Os/Ru
atoms and the substrate
W
atoms becomes rapid. The emission characteristics
of M-type cathodes asymptotically approach that of B-
type cathodes. At an emission density above
10
A/cm2,
both E-type and M-type cathodes have
too
short a life
expectancy to be of practical use. A dispenser cathode
with a lower work function such as the scandate cath-
ode is needed. However, further developments must be
made before scandate cathodes find wide applications.
One of the major obstacles
is
its susceptibility
to
ion
damage.
Ion
bombardment could cause emission deg-
radation through sputter removal of the surface atoms.
With other types of dispenser cathodes, heating to a
higher temperature completely restores the original
emission characteristics.
It
was found that thermal
treatments were unable
to
reactivate scandate cathodes
after ion beam damage because the lost surface scan-
dium atoms were not readily replenished. A method of
replenishing scandium must be developed and incor-
porated in the cathode fabrication for scandate cath-
odes
to
have a reliable performance in a practical tube
environment.
Secondary Emission
When the surface of a solid is bombarded by
charged particles having appreciable velocity, elec-
trons
are emitted from the solid. This is the process of
secondary emission.$$"#
In
the case where the bom-
barding particles are also electrons, which is the focus
of this discussion, the incident and emitted electrons
are
differentiated by the terms primary and secondary,
respectively. Secondary emission processes under
electron bombardment play an essential role in vac-
uum electronic devices. The materials used in the
devices may need to be judiciously selected in some
cases to enhance the secondary emission or in other
cases
to
suppress such emission. High secondary-
emission materials are desirable for grids in electron
multipliers and for cathodes in crossed-field devices,
such as magnetrons and crossed-field amplifiers.
In
contrast, low secondary-emission materials are desir-
able for depressed collectors in microwave- and milli-
meter-wave power tubes to ensure a high efficiency in
the energy conversion. Low-emission materials are
also sought for coating the grids and tube walls to pre-
vent
RF
vacuum breakdown.
$
H.
Bruining,
Physics and Applications
of
Secondary
Electron Emission
(New York McGraw-Hill Book
Co.,
1954).
0
0.
Hackenberg and
W.
Brauer,
Secondary Electron
Emission from Solids,
Advances in Electronics and Electron
Physics, Vol.
XI
(New York Academic Press, 1959).
''
A.
J.
Dekker,
Secondary Electron Emission,
Solid State
Physics, Vol. 6 (New York: Academic Press, 1958).
#
R.
0.
Jenkins and
W.
G.
Trodden,
Electron and Ion
Emission
(New York Dover, 1965)
p.
54.
