Dinc Ibrahim. Refrigeration systems and applications 2th edition
Подождите немного. Документ загружается.

Advanced Refrigeration Cycles and Systems 231
Table 5. 1 Various properties and performance parameters of the cycle in Figure 5.9 for T
1
= 25
◦
C,
P
1
= 1 atm (0.101 MPa), and P
2
= 20 MPa. The fluid is air.
h
1
= 298.4kJ/kg
h
2
= 263.5kJ/kg
h
3
= 61.9kJ/kg
h
4
= 61.9kJ/kg
h
5
= 78.8kJ/kg
h
6
=−126.1kJ/kg
h
f
=−126.1kJ/kg
s
1
= 6.86 kJ/kg · K
s
2
= 5.23 kJ/kg · K
s
f
= 2.98 kJ/kg · K
T
4
=−194.2
◦
C
x
4
= 0.9177
y = 0.0823
q
L
= 34.9 kJ/kg gas
q
L
= 424 kJ/kg liquid
w
actual
= 451 kJ/kg gas
w
actual
= 5481 kJ/kg liquid
w
rev
= 733 kJ/kg liquid
COP
actual
= 0.0775
COP
rev
= 0.578
η
ex
= 0.134
Table 5. 2 Performance parameters of a simple Linde–Hampson cycle for various fluids.
Item Air Nitrogen Oxygen Argon Methane Fluorine
Liquefaction temperature T
4
(
◦
C) −194.2 −195.8 −183.0 −185.8 −161.5 −188.1
Fraction liquefied y 0.0823 0.0756 0.107 0.122 0.199 0.0765
Refrigeration effect q
L
(kJ/kg gas) 34.9 32.6 43.3 33.2 181 26.3
Refrigeration effect q
L
(kJ/kg liquid) 424 431 405 272 910 344
Work input w
in
(kJ/kg gas) 451 468 402 322 773 341
Work input w
in
(kJ/kg liquid) 5481 6193 3755 2650 3889 4459
Minimum work input w
rev
(kJ/kg liquid) 733 762 629 472 1080 565
COP
actual
0.0775 0.0697 0.108 0.103 0.234 0.0771
COP
rev
0.578 0.566 0.644 0.576 0.843 0.609
Exergy efficiency η
ex
(%) 13.4 12.3 16.8 17.8 27.8 12.7
Here, T represents the temperature of the gas being liquefied in Figure 5.10, which changes
between T
1
and T
6
during the liquefaction process. An average value of T may be obtained using
Equation 5.16 with COP
rev
= 0.578 and T
0
= 25
◦
C, yielding T =−156
◦
C. This is the temperature
a heat reservoir would have if a Carnot refrigerator with a COP of 0.578 operated between this
reservoir at −156
◦
C and another reservoir at 25
◦
C. Note that the same reservoir temperature T
could be obtained by writing Equation 5.15 in the form
w
rev
=−q
L
1 −
T
0
T
(5.17)
where q
L
= 424 kJ/kg, w
rev
= 733 kJ/kg, and T
0
= 25
◦
C.
As part of the analysis, the effects of liquefaction temperature and gas inlet temperature on
various energy- and exergy-based performance parameters are investigated considering oxygen as
the gas being liquefied. The results of these studies are given in Figures 5.11 through 5.18. The
results involving various gases are shown in Figures 5.14 and 5.18.
The data obtained for various fluids in Table 5.2 show that different gases exhibit different
behaviors in terms of performance parameters. The differences are due to the thermophysical
properties of fluids and the liquefaction temperatures. Figures 5.11 through 5.18 show that as
232 Refrigeration Systems and Applications
−180 −170 −160 −150 −140
0.106
0.108
0.11
0.112
0.114
0.116
0.118
0.12
0.122
1000
1500
2000
2500
3000
3500
4000
T
liq
(°C)
y
w
actual
(kJ/kg)
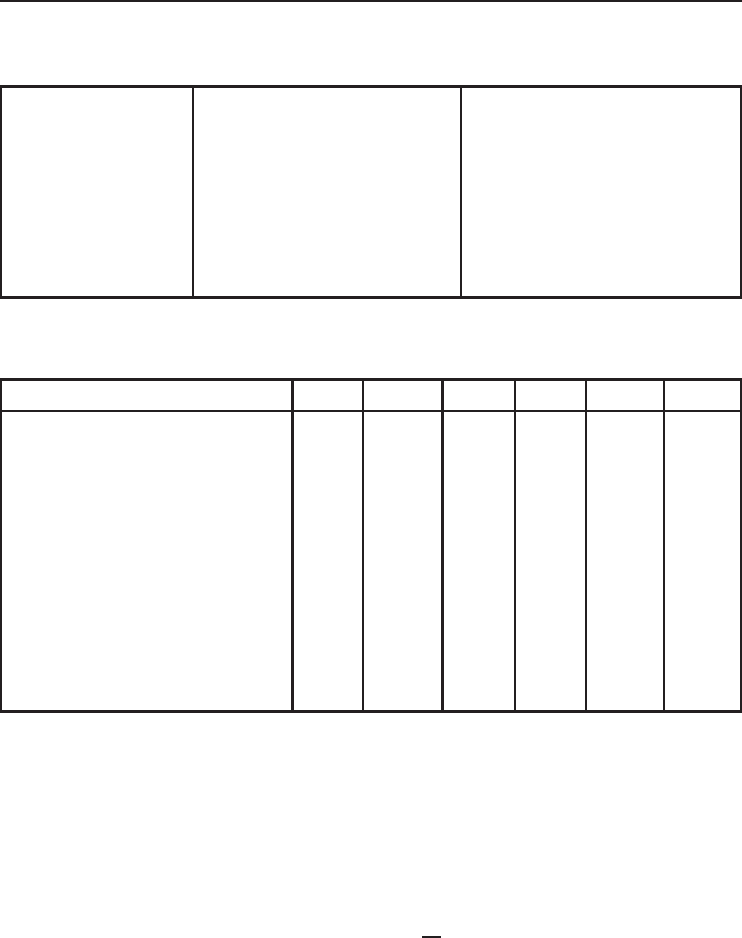
Figure 5.11 The liquefied mass fraction y and the actual work input versus liquefaction temperature for
oxygen.
−180 −170 −160 −150 −140
0.1
0.12
0.14
0.16
0.18
0.2
0.22
0.24
0.6
0.7
0.8
0.9
1
1.1
1.2
COP
actual
COP
rev
T
liq
(°C)
Figure 5.12 The actual and reversible COPs versus liquefaction temperature for oxygen.
Advanced Refrigeration Cycles and Systems 233
−180 −170 −160 −150 −140
0.16
0.165
0.17
0.175
0.18
0.185
0.19
250
300
350
400
450
500
550
600
650
T
liq
(°C)
h
ex
w
rev
(kJ/kg)
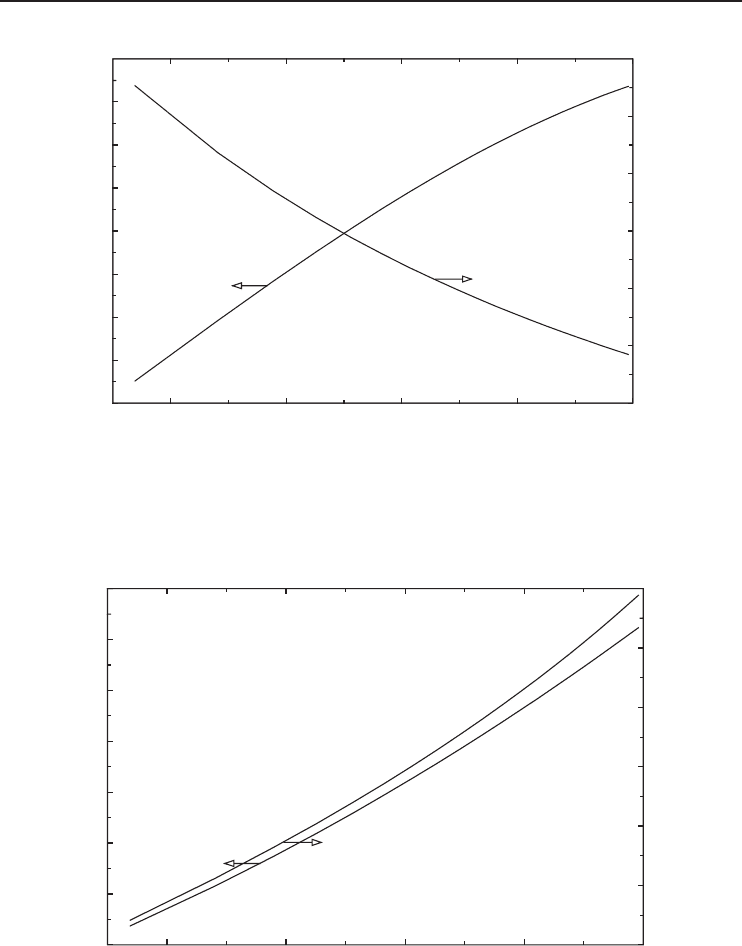
Figure 5.13 The exergy efficiency and reversible work versus liquefaction temperature for oxygen.
−200 −190 −180 −170 −160 −150 −140
0.12
0.13
0.14
0.15
0.16
0.17
0.18
0.19
0.2
T
liq
(°C)
h
ex
Oxygen
Nitrogen
Fluorine
Argon
Air
Figure 5.14 The exergy efficiency versus liquefaction temperature for various gases.
234 Refrigeration Systems and Applications
0 5 10 15 20 25
0.105
0.11
0.115
0.12
0.125
0.13
0.135
0.14
2900
3000
3100
3200
3300
3400
3500
3600
3700
3800
y
w
actual
(kJ/kg)
T
gas
(°C)
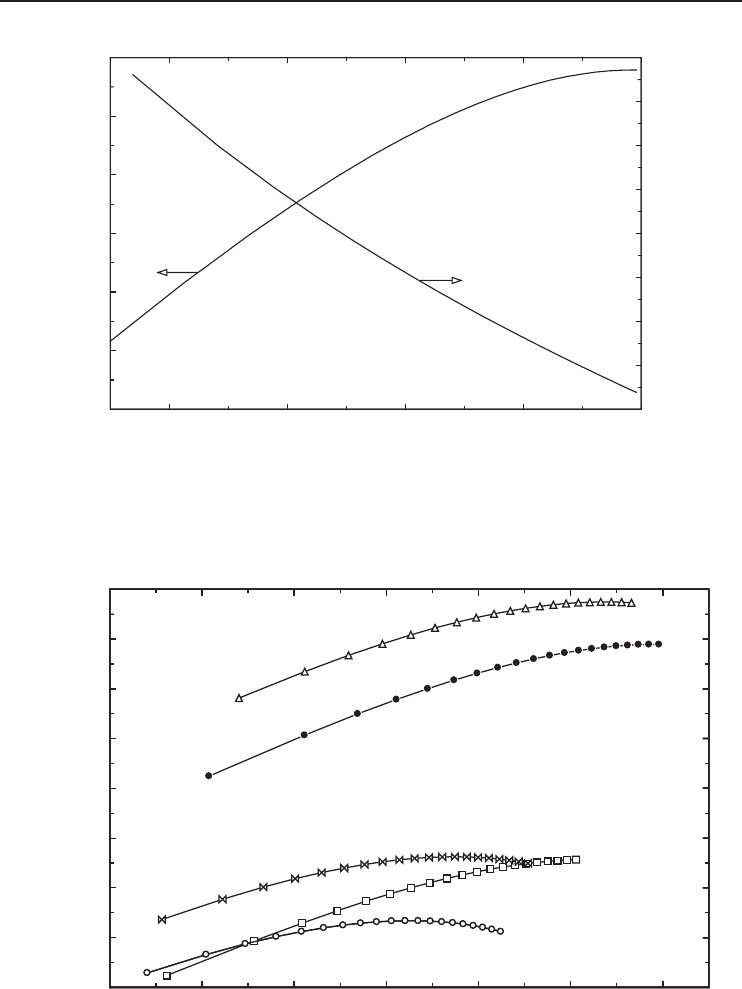
Figure 5.15 The liquefied mass fraction y and the actual work input versus gas inlet temperature for oxygen.
0 5 10 15 20 25
0.105
0.11
0.115
0.12
0.125
0.13
0.135
0.605
0.61
0.615
0.62
0.625
0.63
0.635
0.64
0.645
COP
COP
rev
T
gas
(°C)
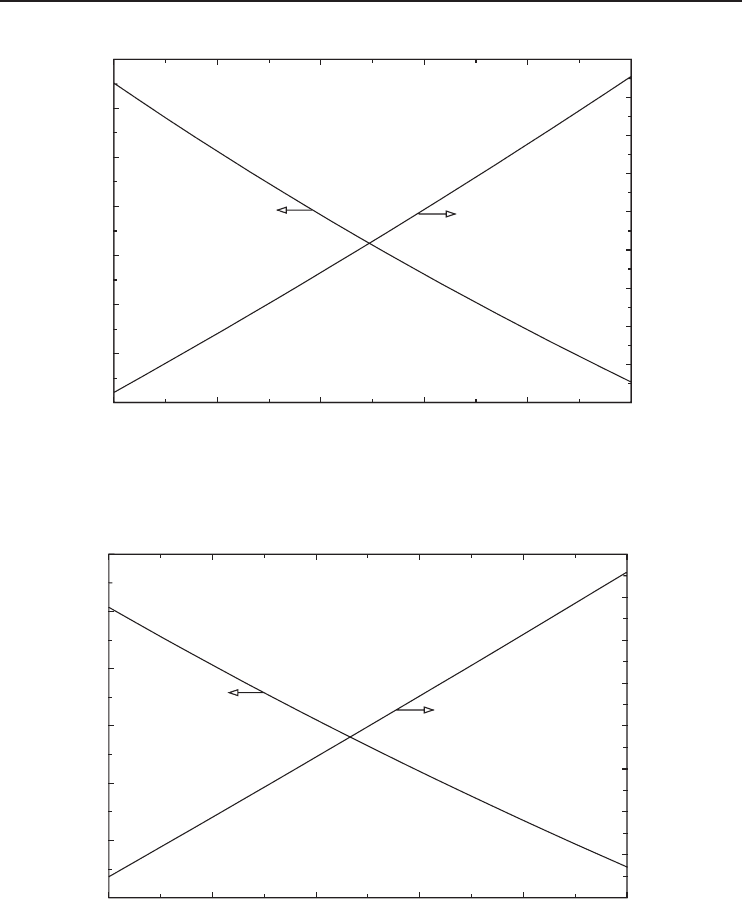
Figure 5.16 The actual and reversible COPs versus gas inlet temperature for oxygen.
Advanced Refrigeration Cycles and Systems 235
0 5 10 15 20 25
0.16
0.17
0.18
0.19
0.2
0.21
0.22
628
628.2
628.4
628.6
628.8
629
629.2
h
ex
w
rev
(kJ/kg)
T
gas
(°C)
Figure 5.17 The exergy efficiency and reversible work versus gas inlet temperature for oxygen.
0 5 10 15 20 25
0.12
0.14
0.16
0.18
0.2
0.22
h
ex
T
gas
(°C)
Fluorine
Nitrogen
Air
Oxygen
Argon
Figure 5.18 The exergy efficiency versus gas inlet temperature for various gases.

236 Refrigeration Systems and Applications
the liquefaction temperature increases and the inlet gas temperature decreases the liquefied mass
fraction, the actual COP, and the exergy efficiency increase, while actual and reversible work
consumptions decrease.
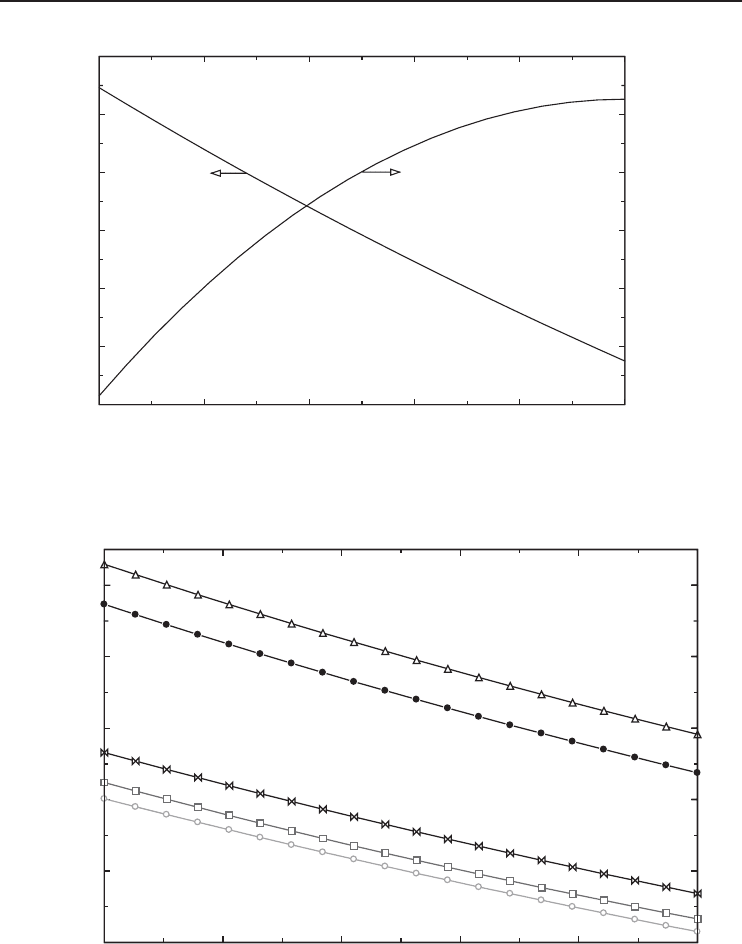
It is interesting to observe from Figure 5.16 that the reversible COP increases as the gas inlet
temperatures increase. This unexpected trend is due to the fact that the refrigeration effect increases
at a greater rate than the reversible work input when the inlet gas temperature increases. On the
other hand, the reversible COP increases as the liquefaction temperature increases as shown in
Figure 5.12 because of the fact that the reversible work input decreases at a greater rate than the
refrigeration effect.
The exergy efficiency increases with increasing liquefaction temperature and decreasing inlet
gas temperature for all gases considered as shown in Figures 5.14 and 5.18. In Figure 5.14, the
exergy efficiency reaches a maximum before decreasing at higher temperatures. The decreasing
trend at higher liquefaction temperatures is of no practical importance since liquefaction at these
high temperatures requires higher inlet pressures, which are not normally used.
Obtaining liquefied oxygen at −183
◦
C requires exactly 2.1 times the minimum work required to
obtain oxygen at −145
◦
C (Figure 5.13). This ratio becomes 2.4 when actual work consumptions
at these temperatures are considered (Figure 5.11). Similarly, the reversible COP decreases almost
by half when the liquefaction temperature decreases from −140 to −190
◦
C (Figure 5.12). These
figures show that the maximum possible liquefaction temperature should be used to minimize the
work input. In another words, the gas should not be liquefied to lower temperatures than needed.
As the inlet gas temperature decreases from 25 to 0
◦
C, the actual specific work input decreases
from 3755 to 2926 kJ/kg (Figure 5.15). The reversible work is not notably affected by the inlet gas
temperature (Figure 5.17).
Among the results provided in Figures 5.11 through 5.18, the exergy efficiency values and trends
appear to provide the most valuable information by clearly showing that the system performance
increases with increasing liquefaction and decreasing inlet gas temperatures and that there is a
significant potential for improving performance. Among the gases considered, argon performs best
while nitrogen performs worst (Figures 5.14 and 5.18). Noting that the cycle considered in this
example involves a reversible isothermal compressor and a 100% effective heat exchanger, the
exergy efficiency figures here are better than what they would be for an actual Linde–Hampson
cycle. In practice, an isothermal compression process may be approached by using a multistage
compressor. For higher effectiveness, a larger and thus more expensive heat exchanger would
be needed. The work consumption may be decreased by replacing the expansion valve with a
turbine. Expansion in a turbine usually results in a lower outlet temperature relative to that for an
expansion valve while producing work, thus decreasing the total work consumption in the cycle.
The complexity and added cost associated with using a turbine as an expansion device is only
justified in large liquefaction systems (Kanoglu, 2001). In some systems both a turbine and an
expansion valve are used to avoid problems associated with liquid formation in the turbine.
The system considered in this study involves an ideal isothermal compressor and a perfect heat
exchanger with an effectiveness of 100%. When a more realistic cycle for air liquefaction with an
isothermal efficiency of 70% and a heat exchanger effectiveness of 96.5% is analyzed, the liquefied
mass fraction decreases by about 22% and the work consumption increases by 1.8 times compared
to ideal cycle. The actual exergy efficiencies of Linde–Hampson liquefaction cycle are usually
under 10% (Barron, 1985).
The difference between the actual and reversible work consumptions in liquefaction systems
are because of the exergy losses that occur during various processes in the cycle. Irreversible
compression in the compressor, heat transfer across a finite temperature difference in heat exchang-
ers (e.g., regenerator, evaporator, compressor), and friction are major sources of exergy losses
in these systems. In actual refrigeration systems, these irreversibilities are normally reduced by
applying modifications to the simple Linde–Hampson cycle, such as utilizing multistage compres-
sion and using a turbine in place of an expansion valve or in conjuction with an expansion valve
Advanced Refrigeration Cycles and Systems 237
(Claude cycle). Other modified cycles that have resulted in greater efficiency are known as the dual-
pressure Claude cycle and the Collins helium cycle. For natural gas liquefaction, mixed-refrigerant,
cascade, and gas-expansion cycles are used (Kanoglu, 2002). In most large natural gas liquefaction
plants, the mixed-refrigerant cycle is used in which the natural gas stream is cooled by the suc-
cessive vaporization of propane, ethylene, and methane. Each refrigerant may be vaporized at two
or three pressure levels to minimize the irreversibilities and thus increase the exergy efficiency of
the system. This requires a more complex and costly system but the advantages usually more than
offset the extra cost in large liquefaction plants.
5.4.2 Precooled Linde–Hampson Liquefaction Cycle
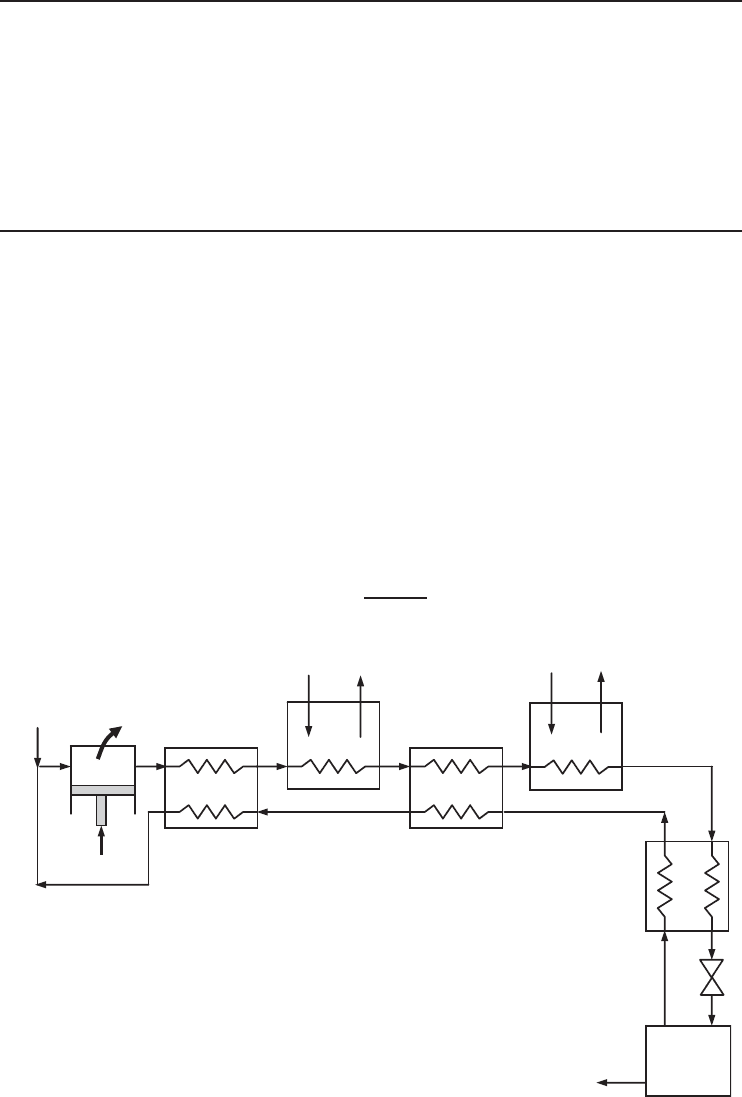
The precooled Linde–Hampson cycle is a well-known and relatively simple system used for the
liquefaction of gases including hydrogen (Figure 5.19). Makeup gas is mixed with the uncondensed
portion of the gas from the previous cycle and the mixture at state 1 is compressed to state 2.
Heat is rejected from the compressed gas to a coolant. The high-pressure gas is cooled to state 3
in a regenerative counter-flow heat exchanger (I) by the uncondensed gas and is cooled further by
flowing through two nitrogen baths (II and IV) and two regenerative heat exchangers (III and V)
before being throttled to state 8, where it is a saturated liquid–vapor mixture. The liquid is collected
as the desired product and the vapor is routed through the bottom half of the cycle. Finally, the
gas is mixed with fresh makeup gas and the cycle is repeated.
Using an energy balance of heat exchanger V and the throttling valve taken together, the fraction
of the liquefied gas can be determined to be
f
liq
=
h
9
− h
6
h
9
− h
f
(5.18)
W
Q
m
f
m−m
f
..
1
2
m
34
5
6
7
8
9
g
10
11
I
II
III
IV
V
Liquid
LN
2
GN
2
LN
2
GN
2
Makeup
gas
f
.
.
.
.
Figure 5.19 Precooled Linde–Hampson liquefaction cycle.
238 Refrigeration Systems and Applications
Energy balances for the heat exchangers can be written as
h
2
− h
3
= (1 − f
liq
)(h
11
− h
10
) (5.19)
h
4
− h
5
= (1 − f
liq
)(h
10
− h
9
) (5.20)
h
6
− h
7
= (1 − f
liq
)(h
9
− h
g
) (5.21)
Since the gas behaves ideally during compression, the specific compression work may be deter-
mined from
w
in
=
RT
0
ln(P
2
/P
1
)
η
comp
(per unit mass of gas in the cycle) (5.22)
where η
comp
is the isothermal efficiency of the compressor, R is the gas constant, and P is the
pressure. The numerator of the right side represents the work input for a corresponding isothermal
process. The specific work input to the liquefaction cycle per unit mass of liquefaction is
w
in,liq
=
w
in
f
liq
(per unit mass of liquefaction) (5.23)
Example 5.3
Hydrogen gas at 25
◦
C and 1 atm (101.325 kPa) is to be liquefied in a precooled Linde–Hampson
cycle. Hydrogen gas is compressed to a pressure of 10 MPa in the compressor which has an
isothermal efficiency of 65%. The effectiveness of heat exchangers is 90%. Determine (a) the
heat removed from hydrogen and the minimum work input, (b) the fraction of the gas liquefied,
(c) the work input in the compressor per unit mass of liquefied hydrogen, and (d) the second-law
efficiency of the cycle if the work required for nitrogen liquefaction is 15,200 kJ/kg of hydrogen
gas in the cycle. Properties of hydrogen in the cycle at various states are as follows:
h
f
= 271.1kJ/kg
h
0
= 4200 kJ/kg
h
6
= 965.4kJ/kg
h
9
= 1147.7kJ/kg
s
f
= 17.09 kJ/kg · K
s
0
= 70.42 kJ/kg · K
Solution
(a) The heat rejection from hydrogen gas is
q
L
= h
0
− h
f
= (4200 − 271.1) kJ/kg = 3929 kJ/kg
Taking the dead state temperature to be T
0
= T
1
= 25
◦
C = 298.15 K, the minimum work
input is determined from
w
min
= h
0
− h
f
− T
0
(s
0
− s
f
)
= (4200 − 271.1) kJ/kg − (298.15 K)(70.42 − 17.09) kJ/kg · K
= 11,963 kJ/kg
Advanced Refrigeration Cycles and Systems 239
(b) The fraction of the gas liquefied is
f
liq
=
h
9
− h
6
h
9
− h
f
=
1147.7 − 965.4
1147.7 − 271.1
= 0.208
(c) The work input in the compressor per unit mass of hydrogen gas compressed is
w
in
=
RT
0
ln(P
2
/P
1
)
η
comp
=
(4.124)(298.15) ln(10,000/101.325)
0.65
= 8682 kJ/kg
Per unit mass of liquefaction,
w
in,liq
=
w
in
f
liq
=
8682
0.208
= 41,740 kJ/kg
(d) The total work input for the cycle per unit mass of liquefied hydrogen is
w
in,total
=
w
in
+ w
in,nitrogen
f
liq
=
8682 + 15,200
0.208
= 114,800 kJ/kg
(e) The second-law efficiency is determined from
η
II
=
w
min
w
in,total
=
11,963
114,800
= 0.104 = 10.4%
5.4.3 Claude Cycle
Claude cycle may be used to liquefy various gases including hydrogen (Figure 5.20). In this cycle,
an expander (turbine) makes work production during the expansion process possible. Feed gas
is compressed to approximately 40 bar pressure. About 75% of the gas after the primary heat
exchanger is expanded in a turbine before mixing with the cold returning gas. An expansion valve
is used to obtain liquid. An energy balance on the entire cycle with no heat leak into the cycle
gives
( ˙m −˙m
f
)h
1
+˙m
f
h
f
+˙m
e
h
e
−˙mh
2
−˙m
e
h
3
= 0 (5.24)
The fraction of mass flowing through the expander is defined as
x =˙m
e
/ ˙m
f
(5.25)
Then the fraction of mass liquefied becomes
y =
˙m
f
˙m
=
h
1
− h
2
h
1
− h
f
+ x
h
3
− h
e
h
1
− h
f
(5.26)
The last term is greater than zero and thus Claude cycle is a definite improvement over the
Linde–Hampson cycle. The work produced in the expander is given by
W
e
=˙m
e
(h
3
− h
e
) (5.27)
The total work input is the difference between the work consumed in the compressor and the
work produced in the expander:
w = w
comp
− w
e
=
[
T
1
(s
1
− s
2
) − (h
1
− h
2
)
]
− x(h
3
− h
e
) (5.28)
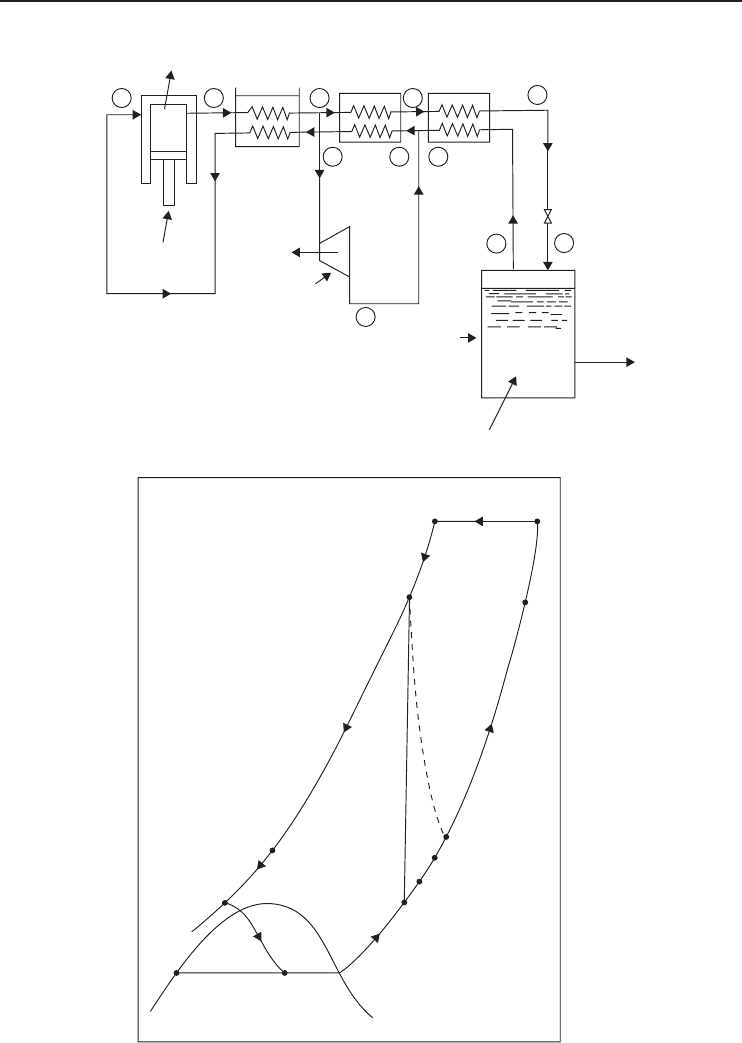
240 Refrigeration Systems and Applications
Liquid
Q
r
m
m
e
.
.
.
.
W
e
.
Q
a
.
W
e
12 3
987
m
f
e
4
5
6
g
Expander
Evaporator
Temperature T
Entropy s
g
f
6
7
8
5
4
e
h = const
s = const
p = const
p = const
3
9
1
2
T = const
(b)
(a)
Figure 5.20 A Claude low-pressure process cycle using an (a) expansion machine and (b) its T − s diagram
(Adapted from Barron, 1985).
